REGULATORY & QUALITY COMPLIANCE SERVICES
Navigating regulatory affairs and quality compliance (RAQA) with FDA regulations and international standards can be perplexing, leading to delayed projects and timeframes when done incorrectly. Non-compliance may bring about even bigger problems. As a result, regulatory agencies demand manufacturers maintain compliance throughout all stages of a product’s lifecycle, from concept through post-market compliance. Square 1 ’s RAQA Services (Regulatory, Quality Assurance & Quality Control) offer a practical yet methodical approach to regulatory compliance and control processes to maintain quality from development through the patient experience.
REGULATORY SERVICES:
Regulatory Strategy
Product Gap Analysis & Remediation
FDA/Agency Meeting Preparation
Face-to-Face Representation at Agency Meetings
IDE, 510(K), PMA Preparation & Submissions
Device Labeling
Complaint Handling System Implementation & Maintenance
Compliance SOP Development
Product Gap Analysis & Remediation
FDA/Agency Meeting Preparation
Face-to-Face Representation at Agency Meetings
IDE, 510(K), PMA Preparation & Submissions
Device Labeling
Complaint Handling System Implementation & Maintenance
Compliance SOP Development
QUALITY ENGINEERING SERVICES:
Design Control Process/Design and Implementation
Quality Systems Design & Implementation
Statistical Experimental Design
Risk Analysis
Process and Test Method Development
Software Development/ Systems Integration
Process/ Product Quality Issue Resolution (CAPA/ NCMR)
MDD to MDR Transition
Verification & Validation
Cost Reduction Strategies
Process Optimization/ Improvement
Technology Transfer
Process Automation
Production Equipment Relocation
Training & Education
Program & Project Management
Supplier Acquisition & Assessments
Document Control
Quality Systems Design & Implementation
Statistical Experimental Design
Risk Analysis
Process and Test Method Development
Software Development/ Systems Integration
Process/ Product Quality Issue Resolution (CAPA/ NCMR)
MDD to MDR Transition
Verification & Validation
Cost Reduction Strategies
Process Optimization/ Improvement
Technology Transfer
Process Automation
Production Equipment Relocation
Training & Education
Program & Project Management
Supplier Acquisition & Assessments
Document Control
INSPECTION READINESS SERVICES:
Simulated Inspections
ISO, MDD, MDR, MDSAP Audit
Risk-based Preparation
Inspection Oversight and Management
Experiential Learning for SMEs
483/Warning Letter Responses
Training & Education
ISO, MDD, MDR, MDSAP Audit
Risk-based Preparation
Inspection Oversight and Management
Experiential Learning for SMEs
483/Warning Letter Responses
Training & Education
IS MEDTECH COMPLIANCE WORK HOLDING YOU BACK?
|
Meet Frank! He's overwhelmed with device compliance work - sound familiar? Don't worry Frank, there's a solution to your problems...
|
|
Remediation Case Study |
PRODUCT EXPERIENCE: |
|
Our client, a Class III medical device company, received multiple FDA 483’s as a result of Class-III product recall due to several system wide nonconformances, both major and minor, ranging from QMS, Operations, and R&D Sustaining.
Read the full details via the attached case study below.
|
Class I, II (a & b), III
Delivery Devices Diagnostics Disposables Endovascular Neurovascular (stents, coils, catheters) Ophthalmics (IOLs & ICLs) Plastic Injection Molded Components Sensing & Monitoring Systems Surgical Instrumentation Vision Systems Wellness Devices | ||||||
SQUARE-1 CAPABILITIES INFORMATION
Square-1 Engineering offers support in a variety of medical device project disciplines across R&D, RAQA and Manufacturing Engineering. Learn how we engage in each of those phases by clicking on the icon links below covering Square-1 Engineering case studies, medical device industry and project expertise.
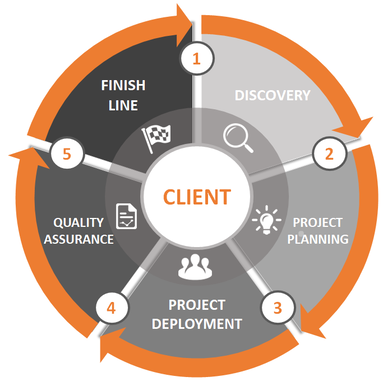
How We Ensure Successful Project Execution
|
Our clients enjoy working with us because of our proven ability to deliver results beyond their expectation using a systematic, yet flexible approach to project engagement, delivery and successful closure.
Learn more about our project engagement process: |
Request Information
Visit Square-1's
|
|