|
Medtech Snapshot Part 2:2 continues the discussion as Trisha Aure covers considerations when transitioning from 21 CFR 820 to ISO 13485.
Hear how due diligence and training play a crucial role in the transition process and the impact it has on our employees. #iso13485 #regulation #compliance #fda #21CFR #riskmanagement #medicaldevice #medtech #news #podcast #snapshot #regulatoryaffairs
0 Comments
In this 2-part series of Medtech Snapshot we're joined by Square-1 Engineering Director of Delivery & Operations Trisha Aure as she walks us through the highlights of our article 'FDA Announcement: 21 CFR 820 and ISO 13485 Guidance'
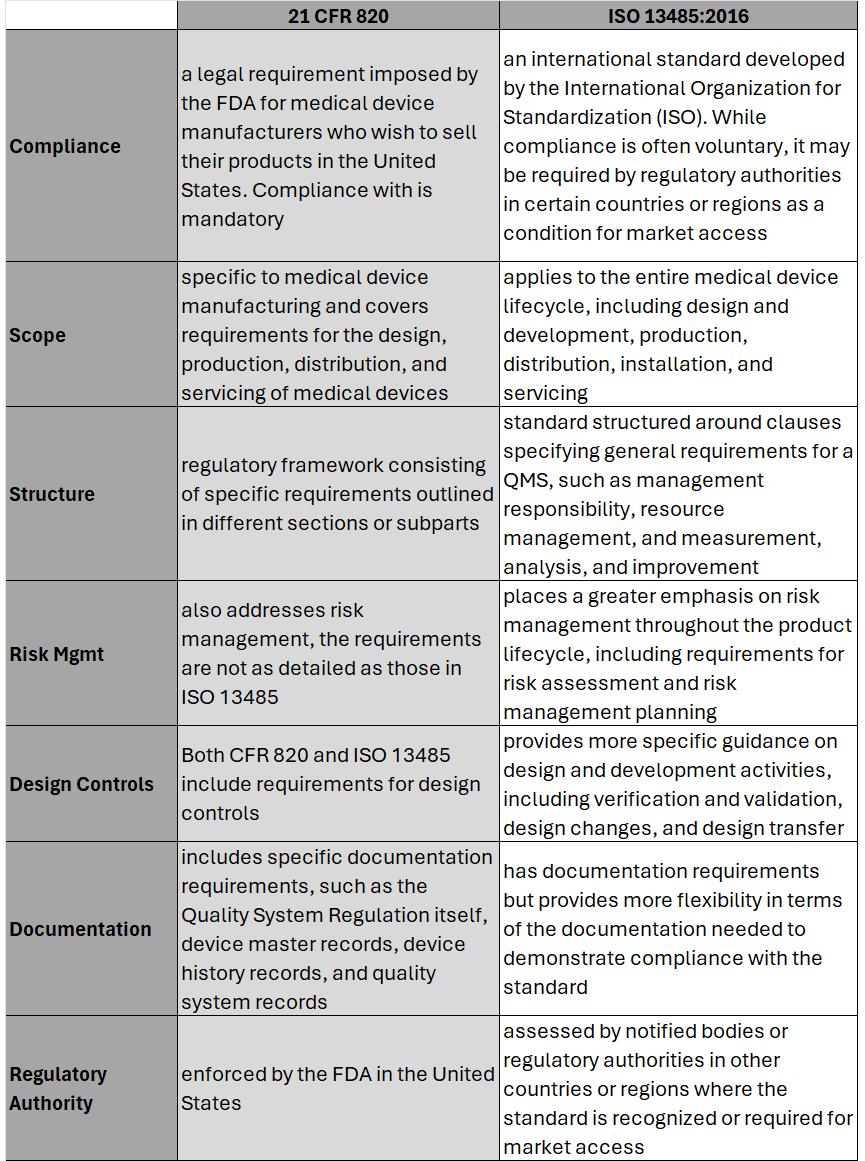
Hear the areas where 21 CFR 820 differs most from ISO 13485 and what this means for medical device OEMs. Part-2 of this series will cover the process to transition and key considerations when doing so. #iso13485 #regulation #compliance #fda #21CFR #riskmanagement #medicaldevice #medtech #news #podcast #snapshot #regulatoryaffairs On February 2nd, 2024 the U.S. Food and Drug Administration (FDA) made its formal announcement and ruling providing guidance on 21 CFR Part 820 which up to that point was the standard overseeing medical device quality system regulation and current good manufacturing practices (cGMP) in the United States of America. The big news – 21 CFR 820 will be heavily amended to incorporate ISO 13485 as the leading guidance for Quality Management System Regulation (QMSR) and cGMP. While the news wasn’t a surprise, it does put a final note on the direction the agency intends to take for medical device practices moving forward, especially as it relates to risk management. While many device OEMs already utilize ISO as their leading regulation standard, those who don’t will have two years to adjust to these changes to be in compliance effective February 2nd, 2026. Here’s what you need to know as it relates to the differences between 21 CFR 820 and ISO 13485, as well as considerations OEMs should take into account in order to meet the 2026 deadline. Big Picture:
Key Differences: Transitioning to ISO 13485 Transitioning from CFR 820 to ISO 13485 involves several steps to ensure compliance with the ISO standard. A general outline of the steps an OEM should take to transition may include: 1. Understand the Requirements of ISO 13485: Familiarize yourself with the requirements of ISO 13485. This includes understanding the structure of the standard, its key clauses, and any specific requirements which may differ from CFR 820. (see above table for highlights) 2. Gap Analysis: Conduct a thorough gap analysis to identify the differences between your current quality management system under CFR 820 and the requirements of ISO 13485. This will help you determine what changes, if any, need to be made to your existing processes, procedures, and documentation. This is also a great time to do a review of your QMS tool to determine if it is an appropriate tool for future use. 3. Document Review and Update: Review your existing documentation, including quality manuals, procedures, work instructions, and forms, to ensure they align with the requirements of ISO 13485. Update or create new documents as necessary to meet the standard's requirements. 4. Training and Awareness: Provide training to relevant personnel to ensure they understand the requirements of ISO 13485 and their roles in implementing and maintaining the QMS. This may include training on new procedures, processes, and documentation. 5. Implementation of New Processes: Implement any new processes or procedures required by ISO 13485. This may include processes related to risk management, design and development, purchasing, production, and service provisions. 6. Internal Audits: Conduct internal audits of your QMS to verify compliance with ISO 13485 requirements. Identify any non-conformities and take corrective actions to address them. 7. Management Review: Hold management reviews to evaluate the effectiveness of the QMS and identify opportunities for improvement. Ensure top management is actively involved in the transition process and committed to maintaining the QMS. 8. Certification Audit: BEFORE you consider this step be sure to speak with a regulatory affairs subject matter expert to ensure it is necessary. Once you believe your QMS is fully compliant with ISO 13485, engage a certification body to conduct a certification audit. The audit will assess your organization's compliance with the standard and determine if you are eligible for certification. 9. Address Non-conformities: If any non-conformities are identified during the certification audit, take corrective actions to address them. The certification body will typically require verification that corrective actions have been implemented before issuing the ISO 13485 certificate. 10. Continual Improvement: Continuously monitor and improve your QMS to ensure ongoing compliance with ISO 13485 and to enhance the efficiency and effectiveness of your processes. Although the 21 CFR 820 and ISO 13485 vary in their structure, and at times use different terminology to describe similar concepts, 21 CFR 820 and ISO 13485 are substantially similar in that both prioritize principles such as risk management, design controls, and continual process improvement. It’s possible as organizations begin to look at their current standards and systems, they will find the transition process is not as cumbersome as initially thought. While this is an obvious assumption, it’s important to note regulatory affairs professionals should be counseled throughout this entire process to ensure appropriateness of adoption and change management. Ensuring the smooth progression of a medical device through product development is more than just having a good idea and the money to back it up. In the ever-evolving landscape of healthcare, the development of cutting-edge medical devices demands a strategic and disciplined approach. At the heart of this process lies several key drivers each with their own distinctive value, yet all of which work in harmony bringing us to the conclusion we are hoping for - getting our products successfully through each phase of product development and eventually out the door to patients in need. This article explores the 'key drivers' which will keep your product development efforts successfully moving forward. Let’s begin with looking at what may very well be an obvious statement – what problem are you trying to solve and is it worth solving in the first place. The second portion of this question is key – is the problem you want to solve, via the technology you intend to develop, actually worth solving. Some advice – not all problems are worth solving, especially in the eyes of investors. It’s going to take deep pockets to build a product, upwards of $100 million on average for a PMA device, which is why its so important to be able to confidently answer this first two-part question: what problem are you trying to solve and is it worth solving in the first place. Once you know your technology front and back, understand the macroeconomics associated with it, it’s time to put a well-crafted plan into place to help keep you on track throughout each phase of the development cycle. Here enters project management. Project management plays a critical role in the success of medical device product development as it holds your team accountable to keeping focused on the key strategies which contribute to a seamless progression from concept to market. This should include: Developing A Clear Project Plan A well-defined roadmap is the cornerstone of any successful project as it delineates tasks, milestones, and dependencies. Roles and responsibilities are outlined, laying the groundwork for a collaborative and efficient work environment. Regular updates and adjustments ensure the plan can accommodate change while staying aligned with the overarching project goals. Timely Decision-Making Delays are costly endeavors. Projects which embrace prompt decision-making empower team members to make informed choices within their spheres of expertise. This can minimize bottlenecks, fostering an environment where decisions were made swiftly, keeping the project on a forward trajectory. Risk Management A robust risk management plan provides continuous assessment of potential challenges. Glen Rabito, COO of Nidus Biomedical, advises “identify the top three (3) risks and go tackle those things first. This will allow us to understand the technical and clinical risks” [associated with the development of your technology]. Effective Communication Clear and transparent communication is the lifeblood of successful projects. Regular team meetings facilitate discussions on progress, challenges, and potential solutions, creating an environment where everyone is well-informed and engaged. Resource Allocation Efficient resource allocation is essential for maintaining momentum throughout the development process. Teams which carefully monitor the allocation of human, financial, and technological resources tend to fare better than those who don’t. Also, what should you keep internally as a core competency versus outsource to someone better set up to facilitate a portion of your work. Adaptability Flexibility in the face of change is a hallmark of successful product development efforts. Project plans should be designed to be adaptable allowing the team to accommodate shifts in scope, requirements, or unforeseen economic challenges. Regulatory Compliance Navigating the complex landscape of medical device development requires a keen understanding of regulatory requirements. Staying informed of regulations and incorporating specific milestones related to regulatory submissions in the project plan is crucial to your success in keeping things moving forward. Prototyping and Iterative Development Teams which embrace an iterative development approach are focused on continuous improvement, allowing the team to make necessary adjustments based on user and stakeholder input. The result is a product which meets regulatory standards AND user needs and expectations. Quality Assurance and Testing Ensuring the highest quality standards is non-negotiable. Thorough quality assurance and testing processes should be integrated at every stage, whereas multiple testing iterations are planned for and issues identified during testing are promptly addressed. Documentation and Traceability Many of us love to gloss over this step – yet doing so is a monstrous mistake. A disciplined approach to documentation is critical for accountability and traceability. A team which maintains accurate and up-to-date documentation throughout the project, establishing clear traceability between milestones, design inputs, and verification/validation activities will be better positioned for success down the road, while mitigating project efforts being put on hold. In the intricate dance of medical device development and effective project management is the guiding force which better positions your team and company for success. The strategies outlined above, from meticulous planning to continuous improvement, collectively contribute to the seamless progression of a product and the overarching project. As the healthcare landscape continues to evolve, these principles will remain essential for navigating the complexities of medical device development and delivering innovations which make a lasting impact on patient care. The quickest way to overcome a business challenge is to get help from those who are experienced in besting your beast! The team at Square-1 Engineering is comprised of a variety of technical and project management professionals who are subject matter experts in the areas of NPD, Quality, Compliance and Manufacturing Engineering. Learn more about how we can solve your work and project problems today to get you back on track!
Medical device companies play a critical role in advancing healthcare as their ability to diagnose, monitor, and treat medical conditions allow patients like you and I the opportunity to recover and live longer. Device companies carry a heavy burden on our behalf and that burden starts with product risk. One of the biggest challenges an OEM medical device organization will be faced with is managing risk, especially during the early stages of product development. The integration of risk management into design control (ISO 14971) is essential for identifying, assessing, and mitigating potential risks associated with the design and development of a medical device. Given risk management is a part of nearly every development process, and is a primary focus of all regulatory agencies like the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA), why is it then so many medical device companies struggle with sound risk management strategies? The failure to address risks adequately can lead to a whole host of problems ranging from regulatory non-compliance, compromised patient safety, financial setbacks, and in severe situations criminal prosecution of executives. Needless to say, understanding why medical device companies come up short with their risk management strategy and how you can avoid that for yourself is key to your future success. In this article, we will explore some of the key reasons behind risk management failure. Most Common Risk Failure Factor - Inadequate Understanding of Regulatory Requirements: One of the primary reasons for failure in risk management is an insufficient understanding of the complex and evolving regulatory landscape. Medical device companies must navigate a web of regulations, standards, and guidelines to ensure compliance. Failing to keep abreast of these requirements can result in flawed risk assessments, inadequate risk mitigation measures, and ultimately, regulatory sanctions. Poor Integration of Risk Management into Product Development: Successful risk management should be an integral part of the product development lifecycle. However, some companies make the mistake of treating it as a standalone process rather than integrating it seamlessly into every stage of development. When risk management is an afterthought, essential risks may be overlooked, leading to suboptimal product designs, increased failure rates, and compromised patient outcomes. Lack of Cross-Functional Collaboration: Effective risk management requires collaboration across various departments, including research and development, regulatory affairs, quality assurance, and manufacturing. Failure to establish clear communication channels and encourage cross-functional collaboration can result in siloed decision-making. This lack of coordination can lead to critical risks being underestimated or missed entirely. Insufficient Resources and Expertise: Some medical device companies fail in risk management due to resource constraints and a shortage of expertise. This can manifest in inadequate training for personnel responsible for risk management, insufficient allocation of time and budget, and a lack of access to external expertise. Without the necessary resources, companies may struggle to conduct comprehensive risk assessments and implement effective risk mitigation strategies. Overemphasis on Short-Term Goals: Pressure to meet short-term financial goals can sometimes lead companies to prioritize speed to market over thorough risk analysis. This can result in hasty decision-making and inadequate risk identification and mitigation. Companies need to strike a balance between meeting market demands and ensuring the safety and efficacy of their medical devices in the long run. Failure to Learn from Industry Incidents: The medical device industry has witnessed several high-profile incidents related to product failures and patient harm. Failure to learn from these incidents and implement lessons learned into future risk management strategies can perpetuate the same mistakes. Companies must actively analyze industry incidents, update risk management processes accordingly, and continuously improve their practices. Ineffective Communication with Stakeholders: Communication is crucial in risk management, both internally and externally. Companies that fail to communicate effectively with their stakeholders, including regulatory bodies, healthcare professionals, and patients, may face increased scrutiny and regulatory challenges. Transparency and open communication are essential for building trust and demonstrating commitment to patient safety. In the highly regulated and dynamic field of medical devices, effective risk management is not just a regulatory requirement - it is a fundamental aspect of ensuring patient safety and the success of a company. Understanding the pitfalls that lead to failures in risk management can help medical device companies proactively address these challenges. By prioritizing compliance, integrating risk management into every stage of product development, fostering cross-functional collaboration, and learning from industry incidents, companies can enhance their risk management strategies and contribute to the advancement of healthcare with safe and reliable medical devices. The quickest way to overcome a business challenge is to get help from those who are experienced in besting your beast! The team at Square-1 Engineering is comprised of a variety of technical and project management professionals who are subject matter experts in the areas of NPD, Quality, Compliance and Manufacturing Engineering. Learn more about how we can solve your work and project problems today to get you back on track!
We're breaking the mold!
Medtech Snapshot returns with an enticing debate as #RAQA medtech industry experts Stephanie Rallis-Daw, RAC, CQE, CMDA and Robert Lahaderne, MBA spar on the topic of "When starting a new job/ project, what is the most common medtech compliance shortfall you can expect to encounter at your new company?" New to Medtech Snapshot? Check out our archive of past episodes at https://www.sqr1services.com/white-papers/category/snapshot covering topics in R&D, Quality, Clinical and Manufacturing. Honey for your eyes and ears, friends. #medtech #snapshot #podcast #medicaldevice #compliance #quality #regulatory #documentation #training One of the biggest challenges professionals face when starting a new job is how they navigate ingratiating themselves into the new company and culture they’re now surrounded by. No two companies are alike, which also means practices, processes and procedures can vary wildly from one company to another. How a new employee, including new management, sorts through this can make or break their ability to be received well by their fellow colleagues while having a good career at their new employer. Imagine you’re in your second week of employment and you begin to uncover a variety of compliance issues like a lack of regulatory understanding throughout the company, poor or missing documentation, insufficient training, little to no post-market surveillance processes or a dinosaur quality management system (QMS) that’s holding the company back. Any of these shortcomings can be problematic for an operation, but the presence of several can be detrimental to the company’s short- and long-term success. It can be a precarious situation to come in as the new ‘gal or guy’ and start changing things right away. In fact, this typically doesn’t bode well for those who take the scorched earth strategy making big changes right out the ‘new hire’ gates, regardless of those changes being warranted. So the question begs to be asked – what do you do if you start a new job and quickly uncover problems within the company’s operations, especially if those problems are compliance related? Taking a measured and strategic approach to your new job and how you will handle the current business practice issues you are experiencing is key to your success. Consider the following process:
Remember that every organization is different, and your approach to addressing poor practices will depend on the specific circumstances. Your ultimate goal should be to contribute positively to the organization's growth and improvement while maintaining your professionalism and integrity. The quickest way to overcome a business challenge is to get help from those who are experienced in besting your beast! The team at Square-1 Engineering is comprised of a variety of technical and project management professionals who are subject matter experts in the areas of NPD, Quality, Compliance and Manufacturing Engineering. Learn more about how we can solve your technical work and project problems today to get you back on track.
In most regulated industries remediation is a cost of doing business. Unfortunately the medical device industry is no different. While remediation won’t hit every business, the fact of the matter is as regulations continue to change and or grow more companies will find themselves in a spot where they are having to change their processes and procedures in order to remain in compliance. In 2021 we wrote about the keys to success, identifying six (6) key areas of focus to help one get through remediation and come out on the other end still in tact and moving forward. As the medical device industry continues to evolve, so must our approach to solving problems we face. As such, understanding the reasons why a device company may experience failure as they go through remediation is key to learning from others mistakes so we don’t repeat them when it comes our turn. WHY THINGS GO SIDEWAYS the top 11 reasons why remediation goes wrong for medical device OEMs:
When looking at this list the biggest take away is the starting point. Once it has been determined remediation is necessary, whether through regulatory intervention or internal, understanding what occurred to get us there in the first place is critical. If we misdiagnose the root cause of the problem within our operations our ability to successfully navigate through the rest of remediation is be hampered significantly. For this reason its wise to spend as much time as possible sorting through the cause and effects of your operation to accurately determine the root cause leading to remediation. Rushing this process will inevitably cause unnecessary challenges on the back end. SOLVING THE PROBLEM The quickest way to overcome a business challenge is to get help from those who are experienced in besting your beast! The team at Square-1 Engineering is comprised of a variety of technical and project management professionals who are subject matter experts in NPD, Quality, Compliance (and yes - remediation) and Manufacturing Engineering. Learn more about how we can solve your compliance problems while besting your remediation beast! EU MDR/ IVDR PROPOSED PUSH1/25/2023 DID YOU KNOW On January 6, 2023 the European Commission, a political and regulatory steering committee consisting of a group of 27 Commissioners, known as 'the College', adopted a proposal to give more time to device OEMs to certify medical devices under EU MDR to mitigate the risk of shortages. The proposal, which now needs to be adopted by the European Parliament, could push out MDR requirements several years. Higher risk devices such as pacemakers and joint implants would have a shorter transition period till December 2027, whereas lower risk devices, such as syringes or reusable surgical instruments wouldn't be until December 2028. WHAT DOES THIS MEAN FOR YOU? Regardless of EU Parliament's decision to potentially extend MDR, device OEMs should consider the following as we hedge through 2023: 1. Strategies for US product approval and or commercialization will continue to increase as OEMs seek alternative pathways to potentially avoid EU MDR compliance. 2. As a result of #1, support to aide OEMs in their go-to-market strategy will intensify causing a shortage for resources, while potentially lengthening the process to get to approvals (supply & demand constraints - notified bodies and consulting firms experience increases in demand causing support shortages). This will be especially true with remediation work. 3. The idea of putting off or slowing MDR related efforts in the interim to re-focus on other activities may provide momentary relief, however it also creates a long-term liability in the business. This liability comes with a variety of future unknowns: regulatory landscape, inflation, cost of resources, CRO and notified body constraints, etc. If you must achieve MDR compliance our recommendation is to get it done and over with in the present. 4. Work associated with achieving MDR compliance can be easily underestimated, especially if you have legacy product where your CE mark was granted pre mid 2000s. The burden to meet MDR requirements may be steep, which is all the more reason to avoid procrastinating said efforts as outlined in #4. SOLVING THE PROBLEM The quickest way to overcome a business challenge is to get help from those who are experienced in besting your beast! The team at Square-1 Engineering is comprised of a variety of technical and project management professionals who are subject matter experts in the areas of NPD, Quality, Compliance (and yes - remediation) and Manufacturing Engineering. Learn more about how we can solve your compliance problems while besting your EU MDR beast! Learn about Square-1 Engineering's mission and what it means to be fearless!
Orange County unemployment rate is 2.9 percent as of July, 2022, as such the talent scarcity is creating gaps between the supply and demand of skilled MedTech professionals. Given these challenges we’re all facing we recently asked the online community the following question:
Which part of your business has the hardest time finding and hiring talent? I suppose EU MDR is to blame for this as the majority of respondents indicated RA QA personnel are the hardest to find. There’s another distinction worth noting that’s adding fuel to the fire. The cost of living (COLA) in Orange County is 54% higher than the national average. As a result, this has a direct impact on the sheer number of people who can afford to live in OC, which decreases the size of the employment pool. Add into the mix issues the overall State is experiencing like a 250k net migration loss along with relatively new industry regulations like EU MDR and you have a perfect storm where demand is grossly outpacing supply. Pete Nalbach, GM of SeaSpine in Irvine, CA shared some interesting insights about the present hiring, employment and talent situation: Pete indicated and I’m paraphrasing a bit “…candidates have options. This means they only accept jobs they really want which in turn gets a higher engaged employee for the company in the long term” What’s your solution to the talent shortage? About the AuthorTravis Smith is the founder and managing director of Square-1 Engineering, a medical device consulting firm, providing end to end engineering and compliance services. He successfully served the life sciences marketplace in SoCal for over 15 years and has been recognized as a ‘40 Under 40’ honoree by the Greater Irvine Chamber of Commerce as a top leader in Orange County, CA. Categories
All
Archives
April 2024
|
Visit Square-1's
|
|









 RSS Feed
RSS Feed


