|
Would you willingly spend $3,000.00 for a 10% chance your ‘investment’ would produce a good return? While many of you reading this instinctively thought “absolutely not”, there inevitably was a select few who thought to themselves, “depends on what the return is”. A 10% chance your initial investment produces a positive upside is not a favorable proposition to say the least yet businesses do this all the time, in fact multiple times, if not dozens of times, a year. I welcome you to the flunky world of ‘corporate training’. Skillademia, an online training and stats organization, indicates US companies spend upwards of $200 billion a year on training and said training carries about a 10% effectiveness outcome. The average company spends $3,000 per employee on annual training with dismal results to show for it. Why is it then companies consistently spend so much money on training for such a poor return. Answer – companies are missing the connection between information retention and positive reinforcement. In today’s fast-paced and ever-evolving business landscape, employee training is vital, yet just as important is the methodology and mindset of those involved (trainers, company management and the participants. This article seeks to shed some light on why training fails more than it helps, and how you can ensure the next time you invest in training you’re getting more of a return on your investment. THE FAILURE OF CORPORATE TRAINING There seems to be a never-ending list which offers insights into the reasons corporate training fails to meet the mark, therefore improving employee performance. The #1 reason corporate training fails is due to a lack of reinforcement: Hermann Ebbinghaus, a 19th Century German Psychologist, was a pioneer in the experimental study of memory and discovered ‘The Forgetting Curve’ which correlates a learners ability to retain information over a given time span. Ebbinghaus suggests people forget a large portion of what they learn if there is no reinforcement, as follows:
Other reasons training programs fail, include:
WHAT HAPPENS IF WE DON’T TRAIN After reading the above it could be easy for someone to say “why even make the investment in the first place” as it relates to corporate and employee training. While on face value that may make sense, the reality of the situation is far worse than most of us may realize. Electing to bypass training for your employees can have disastrous results for the company, its ability to operate successfully and its employees. Below are the common pitfalls, and associated consequences of not training employees:
IMPROVE YOUR CHANCES OF CORPORATE TRAINING SUCCESS So what you’re saying is “I’m damned if I do, and damned if I don’t”. While it may seem that way on first blush, there is a silver lining that is well within our reach. The bottom line is, if you aren’t training your employees you simply aren’t a competitive employer. So rather than avoid training all together, the better approach is to be smart about how you engage in the practice of corporate employee training. Below is a quick rundown of the three most important factors to implement to ensure your training dollars are well spent and well received:
TRAINING DONE WELL HAS A HUGE UPSIDE The verdict is in – most of us know training is a good idea, it’s our delivery that needs help. In fact, about 85% of companies with more than 50 employees provide formal training to their employees, and around 95.8% report offering some form of informal training. (InDemand Designer) If you follow the steps above in IMPROVE YOUR CHANCES OF CORPORATE TRAINING SUCCESS, many companies will find training, when done well, has a big upside, including:
Investing in employee training is not just a strategic move, it is a necessity for any organization which aims to thrive in today’s competitive environment. By enhancing skills, boosting morale, improving productivity, fostering innovation, ensuring compliance, promoting a positive culture, and preparing for the future, training provides a myriad of benefits that contribute to the long-term success and sustainability of the organization. Companies that prioritize and continually invest in training will undoubtedly reap the rewards of a skilled, motivated, and forward-thinking workforce. NEED TRAINING SUPPORT?
Square-1 Engineering’s medical device compliance & business operations training courses primary focus is to support the longevity of your operations through knowledge advancement. Hope, is not a strategy for addressing business challenges like poor team performance and product & system nonconformance. Learn how Square-1’s training courses in Solidworks, Design Controls, Risk Management and Root Cause Analysis can propel your teams performance to the next level. Visit us at https://www.sqr1services.com/training.html.
0 Comments
Special thanks to Medical Design & Outsourcing for covering our press release with our new office in Minneapolis Saint Paul (MSP) Minnesota. Stay tuned for more news and updates as we continue to grow our presence and support in MSP. Square-1 Press release link at MDO: https://lnkd.in/gzeCd-Py #pressrelease #expansion #minneapolissaintpaul #minneapolis #medtech #news #medicaldevice #consulting #growth Press Release: Square-1 Engineering, Inc. Expands Service via New Office in Twin Cities, Minnesota5/15/2024
FOR IMMEDIATE RELEASE Square-1 Engineering, Inc. Expands Service via New Office in Twin Cities, Minnesota Saint Paul, MN – 05.21.2024 – Square-1 Engineering, Inc. (Square-1), a leading medical device consulting firm, is excited to announce the opening of its newest office location in the Saint Paul, Minnesota area. This expansion marks a significant milestone for Square-1 as it continues to grow its presence in key markets across the United States, in particular the Twin Cities comprised of greater Minneapolis and Saint Paul (MSP). The decision to establish a presence in MSP comes as a strategic move to better serve clients in the region and meet the growing demand for medical device engineering and compliance work. With its vibrant economy, thriving business community, and talented pool of professionals, the Minneapolis Saint Paul marketplace presents a wealth of opportunity for Square-1 to connect with new clients and partners. "We are thrilled to be opening our new office in Saint Paul to support the MSP marketplace," said Travis Smith, Founder and Managing Director at Square-1. "This expansion underscores our commitment to providing exceptional engineering and compliance consulting services to medtech clients in this dynamic market. We are eager to collaborate with local companies, leveraging our Class II and III medical device expertise to drive success and growth in the areas of new product development, regulatory and compliance and manufacturing engineering." Square-1’s new office will be located in White Bear Lake, Minnesota, situated east just outside the heart of Saint Paul’s downtown business district. The MSP area, in combination with greater Minnesota, represent a rapidly growing market for medical devices as evidenced in the 2023 designation of the ‘Minnesota MedTech Hub 3.0’ (MMT3.0), one of several dozen tech hubs recognized federally across the country which show potential for significant technology growth. According to Minneapolis Saint Paul Regional Economic Development Partnership, “Minnesota is home to 15,000 healthcare organizations which employ more than 469,740 Minnesotans, including the presence of the top 15 medical device companies in the world.” The largest concentration of MMT3.0 can be found within the Twin Cities or Minneapolis Saint Paul (MSP) area. In addition to enhancing client relationships, the Saint Paul office will serve as a regional hub for the Square-1’s project management office, which helped to build the company’s sterling reputation at its headquarters in Southern California. For more information on Square-1 Engineering, Inc and its medical device consulting services and expertise, visit www.square1engineering.com. About Square-1 Engineering: Square-1 Engineering, Inc. a medical device consulting firm, provides end to end technical project services to medical device OEMs and their supply chain partners. Square-1’s services cover projects of all sizes in Product Engineering, Regulatory and Compliance (RAQA) and Manufacturing Engineering. Contact: Square-1 Engineering – The Medtech Problem Solvers Corporate Headquarters 6 Morgan, suite 125 Irvine, CA 92618 www.square1engineering.com [email protected] 844-300-7771 We're thrilled to announce Square-1 Engineering has officially expanded into the Twin Cities, Minnesota marketplace. With this expansion our company is better positioned to support our device customers in the midwest while further expanding our service capabilities.
Learn more about Square-1 HERE. Stay tuned for more information on our growth in the Twin Cities area. Our most recent Medtech Snapshot features product development executive Barry Fulkerson as he takes us through his strategy of obtaining user needs data on the cheap and how creating a patient advisory board can help get you there.
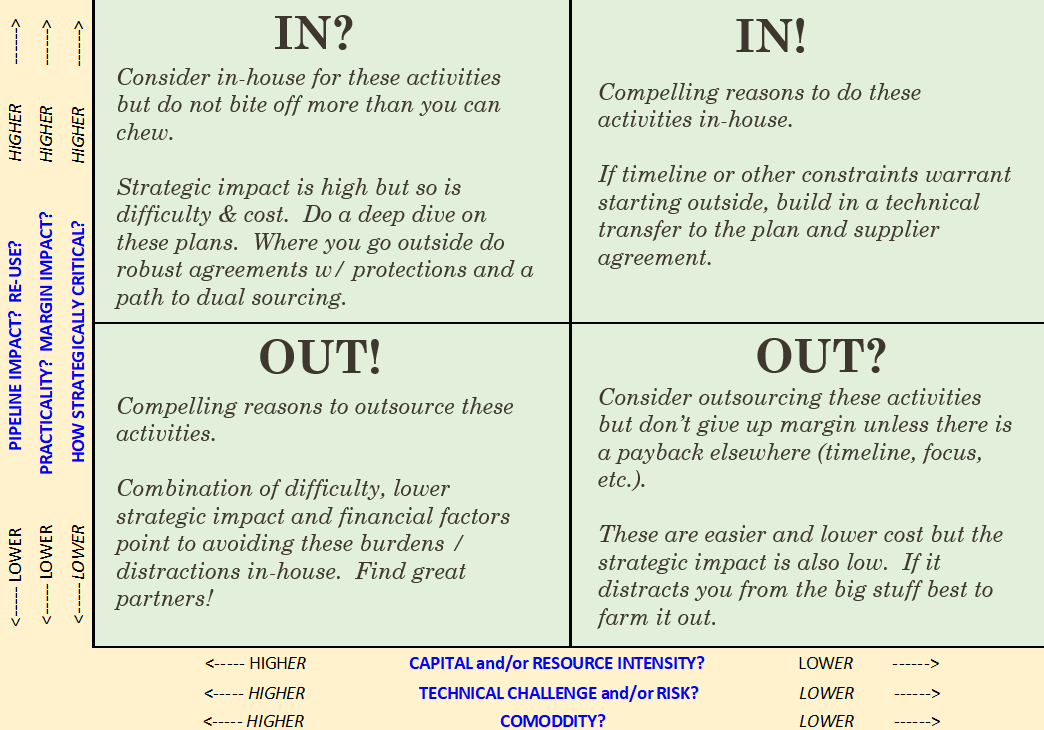
We have archives! To watch and listen in to our past Medtech Snapshot episodes visit https://www.sqr1services.com/white-papers/category/snapshot #userneeds #medtech #snapshot #medicaldevice #productdevelopment #conceptdevelopment #patient In this Medtech Snapshot episode we cover strategies for supplier management and maximizing operational efficiencies when it comes to getting work done. Listen in as Todd Abraham, medical device chief operating officer, explores creative outsourcing, commodity-based decision making and practical manufacturing operation considerations. Need help making a decision on which tasks to keep internally versus to consider outsourcing? Todd has developed the above decision matrix to help guide the process of identifying when it is appropriate to outsource versus insource a particular task. The more we use tools like this to help in our decision making process the better chance we will have of making the right decision, first time around. #suppliermanagement #operations #medicaldevice #medtech #outsourcing #commodity #manufacturing #operations #snapshot The quickest way to overcome a business challenge is to get help from those who are experienced in besting your beast! The team at Square-1 Engineering is comprised of a variety of technical and project management professionals who are subject matter experts in the areas of NPD, Quality, Compliance and Manufacturing Engineering. Learn more about how we can solve your work and project problems today to get you back on track!
Selecting the right medical device contract manufacturing partner is a critical decision that can significantly impact the success of your medical device development and production process. There are many advantages which come with working with a contract manufacturing partner, chief among them is it can potentially help you get out to the market quicker without having to go vertical, building up your own production system internally. The right partner can also offer cost savings as a result of their established supply chain partners, ability to scale and expertise in developing similar products. While those advantages can indeed be a blessing to a maturing business, landing the wrong partner can also be a curse. For this reason its vital device companies utilize a strategically planned process for how they are to approach and select a new contract manufacturing partner. The more due diligence one can do on the forefront the better the likelihood will be you will see success on the back end. Here are some important criteria to consider when evaluating potential contract manufacturing partners: 1. Experience and Expertise: Look for a partner with experience in manufacturing similar medical devices. Their expertise in the specific type of device, materials, processes, and technologies is vital to ensuring a smooth production process and high-quality output. 2. Capabilities and Facilities: Evaluate the contract manufacturer's facilities, equipment, and technological capabilities. Make sure they have the necessary infrastructure and resources to meet your manufacturing needs and scale as your project progresses. (HINT - learn about their employees. If they have high turnover with their internal staff this could create problems for you in the future) 3. Supply Chain Management: A robust supply chain is crucial for consistent component availability and cost-effectiveness. Assess the manufacturer's ability to manage the supply chain efficiently, including sourcing components, managing inventory, and handling potential disruptions. 4. Flexibility and Scalability: Consider the partner's ability to adapt to changes in project scope or production volume. A flexible partner can accommodate adjustments in design, production, and scheduling, which is important in the dynamic field of medical device development. MEDTECH SNAPSHOT: CONTRACT MANUFACTURING In this episode we are joined by Innova Vascular's CEO Sanjay Shiravastava who takes us through several pointers on when to consider finding a medical device contract manufacturing partner to aide in your go to market strategy. Here from Sanjay on why it's "important to begin with the end in mind". 5. Regulatory Compliance: Ensure that the contract manufacturer has a solid understanding of regulatory requirements, such as FDA (or equivalent) regulations, ISO standards (e.g., ISO 13485 for medical devices), and any other relevant local or international regulations. They should have a track record of successfully producing devices that meet these requirements. 6. Quality Management: A strong quality management system is crucial for medical device manufacturing. The partner should have established quality control processes, including inspection, testing, documentation, and corrective action procedures. They should also have a history of adhering to quality standards and maintaining good manufacturing practices. Their data and practices should be transparent and they should be open to you reviewing this information. 7. Geographical Location: The location of the contract manufacturer can impact shipping costs, lead times, and the ease of visiting the facility for inspections or meetings. 8. Intellectual Property Protection: Ensure that the contract manufacturer has protocols in place to protect your proprietary information and intellectual property throughout the manufacturing process. Do they work with a competitor of yours, if so how will they ensure your IP and product is protected? 9. References and Reputation: Research the manufacturer's reputation in the industry. Seek references from other companies they have worked with and review their track record to gauge their reliability, performance, and commitment to quality. 10. Cost and Pricing: While cost should not be the sole deciding factor, it's important to have a clear understanding of the pricing structure, including setup costs, unit costs, and any additional charges. Pricing should be clearly laid out and defined in your contract; stay away from partnerships where things seem murky and or inconsistent from a pricing perspective. 11. Long-Term Partnership Potential: Consider whether the contract manufacturer is interested in building a long-term partnership and is willing to support your future projects and iterations. 12. Employees and Training: Be mindful of how their employees are treated, trained and managed. High turnover at a contract manufacturer may mean you find yourself constantly involved int he training process to ensure the operators and assemblers working on your product are working as expected. The more turnover that occurs the more training that will need to take place, which means your partners training methods and consistently is critical to your success. High turnover and poor training will inevitably lead to yield problems. Before making a final decision, it's advisable to visit the contract manufacturer's facility, meet their team, and discuss your project in detail to ensure alignment and understanding. Taking the time to thoroughly assess potential partners will increase the likelihood of a successful and fruitful collaboration in medical device manufacturing. The world around us is ever-changing and constantly evolving, and for those of us who are leading teams, projects or companies this presents a daily challenge. One area in the business world where we commonly run into change, or scope creep as many of us like to call, is in project work. It doesn’t matter if you are running an internal project, or if you are a consultant operating externally, change in work and projects alike seems constant. If you are new to the term, scope creep is defined as “changes, continuous or uncontrolled growth, in a project’s requirements, at any point after the project begins.” Simply put, it’s a change in plans that was unplanned for after the starting point of a body of work. While complete prevention of scope creep might be unreasonable, employing strategies at the forefront of your project and throughout are vital to ensure you stay on track with as little change along the way. Strategies like the ones listed below also allow the project to ebb and flow so that some change can be accommodated while other changes, which could derail a project, are held off at the proverbial gates. Below you are some strategies to help you prevent scope creep on your next project: 1. Clearly Define the Scope Up Front Have a detailed project scope statement that outlines the project's objectives, deliverables, boundaries, and limitations. Make sure all stakeholders agree on the scope before starting the project. Special note – want to get approval quickly for your project? Make sure the project and intended outcomes align with the company’s mission and primary objectives while clearly showing a good return on investment (ROI). 2. Engage Stakeholders From the Beginning Involve key stakeholders in the scope definition and planning processes. Their input and feedback can help identify potential scope creep early on and ensure that their expectations align with the project's scope. 3. Set Realistic Goals Establish achievable project goals and objectives. Unrealistic expectations can lead to scope creep as stakeholders try to add additional features or requirements. If you can’t get agreement on the goals of the project this should cause you to pause before moving forward. Remember, each person involved may have a different perspective of what is needed based on their own biases. 4. Create a Change Control Process Develop a formalized process for requesting and approving changes to the project scope. I like to use the engineering change order (ECO) process on our projects as it formalizes changes and requires approval from stakeholders before the changes are enacted. All changes should go through this process, involving the evaluation of their impact on time, cost, and resources before being accepted. Speaking of scope creep, in this episode of Medtech Snapshot Terry Vick, Sr. Director Program Management at Shockwave Medical out of Santa Clara, CA talks about some of the strategies in this list which we need to be aware of and consider upfront in the project planning phase into order to limit scope creep on the back end. Plus, the importance of having an internal project sponsor. Back to our list of strategies to help you prevent scope creep… 5. Prioritize Requirements Use techniques like MoSCoW (Must have, Should have, Could have, Won't have), Five Whys or the Analytic Hierarchy Process (AHP) to prioritize project requirements. This helps in focusing on what's essential and avoids unnecessary additions. 6. Track Progress Against Scope Regularly track project progress against the defined scope. Tools like Microsoft Project which build out real-time Gannt charts are incredibly helpful to visualize tasks and progress. This also helps identify any deviations early on and allows for timely corrective actions. 7. Communicate to Your Team Consistently & Clearly Ensuring all team members and stakeholders understand the project's scope and the implications of scope changes is paramount to the success of the project. Effective communication can help prevent misunderstandings that might lead to scope creep. 8. Document Changes to the Project Keep a detailed record of all changes to the project scope, including their rationale and impact. This documentation helps maintain transparency and accountability. 9. Manage Expectations by Providing Project Updates to Stakeholders Continuously manage stakeholder expectations by providing regular updates on project status and any changes to the scope. This can help prevent unrealistic demands and last-minute additions. 10. Institute a Review and Approval Process for Scope Change Institute a formal review and approval process for scope changes. All changes should be evaluated by relevant stakeholders and approved based on their impact. 11. Stay Firm but Flexible While it's important to resist unnecessary scope changes, be open to valid suggestions or changes that genuinely enhance the project's value. Just ensure that these changes go through the proper evaluation and approval processes. If you can’t readily identify the ROI of the change it probably isn’t a good change to consider. 12. Conduct Regular Project Reviews Conduct regular reviews with stakeholders to ensure that the project is meeting their expectations and to address any concerns before they escalate. When facilitating meetings, try to employ Jeff Bezos’ ‘Two Pizza’ rule when it comes to meeting attendees. 13. Manage Dependencies to Avoid Unintended Cross Functional Impact Understand and manage dependencies between project tasks and deliverables. Changes in one area can often impact other parts of the project. Remember that while complete prevention of scope creep might be challenging, these strategies can significantly help to reduce its occurrence and impact on your project. Regular vigilance and proactive management are key to avoiding scope creep from taking over your project. The quickest way to overcome a business challenge is to get help from those who are experienced in besting your beast! The team at Square-1 Engineering is comprised of a variety of technical and project management professionals who are subject matter experts in the areas of NPD, Quality, Compliance and Manufacturing Engineering. Learn more about how we can solve your work and project problems today to get you back on track! In this episode of Medtech Snapshot we hear from Karen Polkinghorne, President of Network Partners Group, on her three strategic considerations for improving your chances of gaining approval with management on the new project, program or initiative you're trying to get under way. Learn how the companies vision and mission, as well as the return on investment (ROI) for your project, plays an important role in the approval process. #medicaldevice #medtech #snapshot #approval #project #program #management #returnoninvestment #vision #mission Get Medical Device Project Support TodayThe quickest way to overcome a business challenge is to get help from those who are experienced in besting your beast! The team at Square-1 Engineering is comprised of a variety of technical and project management professionals who are subject matter experts in the areas of NPD, Quality, Compliance and Manufacturing Engineering. Learn more about how we can solve your work and project problems today to get you back on track!
What we know to be true is root cause analysis (RCA) plays a vital role in the development, manufacturing, and use of medical devices. When we are able to successfully identify and address the underlying causes of failures or errors, using RCA as a tool in your operation helps to improve the safety, reliability, and effectiveness of medical devices. When done correctly, RCA has a significant impact on medical devices by enhancing patient safety, improving design and manufacturing processes, ensuring regulatory compliance, driving product improvement and iteration, mitigating risks, and supporting post-market surveillance efforts. While all of this may sound common sense, what's most important is when RCA is used and done correctly. In this episode of Medtech Snapshot we're joined by Quality Executive Robert Lahaderne to talk Root Cause Analysis (RCA)! Hear how Robert shares his perspective on the impact Root Cause makes to a medical device organization when it is done correct versus when it is done incorrectly and how rushing through FMEAs and hazard analysis creating unnecessary challenges down the road. #rootcauseanalysis #rootcause #medicaldevice #medtech #fmea #hazardanalysis #patientsafety #riskmanagement Get Medical Device Project Support TodayThe quickest way to overcome a business challenge is to get help from those who are experienced in besting your beast! The team at Square-1 Engineering is comprised of a variety of technical and project management professionals who are subject matter experts in the areas of NPD, Quality, Compliance and Manufacturing Engineering. Learn more about how we can solve your work and project problems today to get you back on track! About the AuthorTravis Smith is the founder and managing director of Square-1 Engineering, a medical device consulting firm, providing end to end engineering and compliance services. He successfully served the life sciences marketplace in SoCal for over 15 years and has been recognized as a ‘40 Under 40’ honoree by the Greater Irvine Chamber of Commerce as a top leader in Orange County, CA. Categories
All
Archives
July 2024
|
||||||
Visit Square-1's
|
|











 RSS Feed
RSS Feed


