|
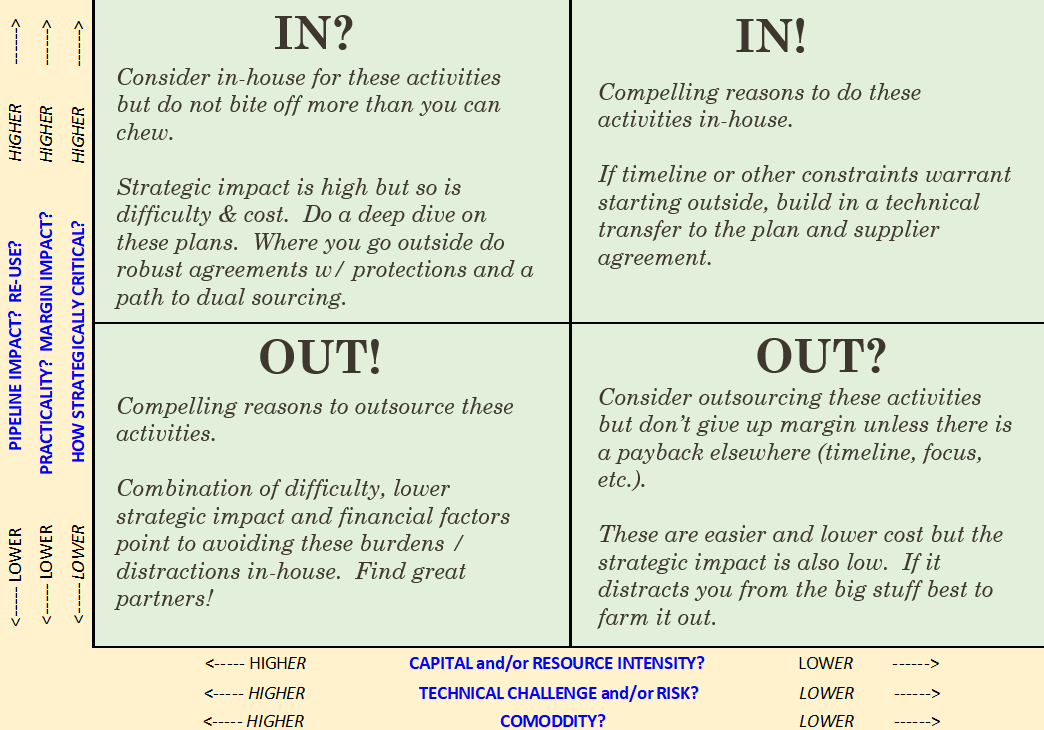
In this Medtech Snapshot episode we cover strategies for supplier management and maximizing operational efficiencies when it comes to getting work done. Listen in as Todd Abraham, medical device chief operating officer, explores creative outsourcing, commodity-based decision making and practical manufacturing operation considerations. Need help making a decision on which tasks to keep internally versus to consider outsourcing? Todd has developed the above decision matrix to help guide the process of identifying when it is appropriate to outsource versus insource a particular task. The more we use tools like this to help in our decision making process the better chance we will have of making the right decision, first time around. #suppliermanagement #operations #medicaldevice #medtech #outsourcing #commodity #manufacturing #operations #snapshot The quickest way to overcome a business challenge is to get help from those who are experienced in besting your beast! The team at Square-1 Engineering is comprised of a variety of technical and project management professionals who are subject matter experts in the areas of NPD, Quality, Compliance and Manufacturing Engineering. Learn more about how we can solve your work and project problems today to get you back on track!
0 Comments
At the forefront of the medical device new product development (NPD) process we typically find a list of considerations we’re trying to address within our product all while coming up with a solution which meets marketplace needs. Talk to anyone in R&D, or downstream marketing for that matter, and you’ll commonly hear the list of ‘needs’ for a new product can seem never-ending. What you may find surprising is how often design for manufacturability (DFM) is not considered in the beginning of the development process. DFM is an important, and often overlooked, part of product development which involves designing a product in a way which makes it easier and more cost-effective to manufacture commercially. While embedding DFM into the process early seems like a logical part of the process, what plays out in reality is an NPD process that is focused on creativity and validating a technology yet can lack appreciation for the future state of the product and it’s foray into the market. So why is it companies may overlook incorporating DFM into the development process? For starters, many of us who design products on the front end, have little perspective, or perhaps no perspective on what it takes to actually produce a scalable product on the back end. Unless you’ve worked on a manufacturing floor or you’ve walked through an entire new product introduction (NPI) cycle, it’s easy to misunderstand all which goes into producing a product to bring to market. The design process also requires creativity and ingenuity in order to find new approaches, break common perceptions of technology capabilities, etc. It’s about ideation! Unfortunately thinking down the road about how it will be built for commercial purposes is “someone else’s problem”. To illustrate this point let’s look towards the automotive industry at a product we all know, which also happens to be a heavily regulated industry, like the medical device industry. If you have ever been to a large-scale automotive show, like the LA Auto Show in Los Angeles, CA, what you’ll see are concept cars on display to wow the eyes and entice the senses. Ironically, if those same concept cars ever got to production, you’ll find their characteristics which made the vehicle look incredible are often diluted down to features and benefits which consumers will accept and are cost efficient to produce. Case in point, the two images below shows a 2010 Chevy Volt electric vehicle. The top image is the concept vehicle – sleek, sophisticated, sharp lines, aggressive character and eye catching. Almost resembles a sports car. The bottom image is the production vehicle – commuter extraordinaire with features which are dull, less angular and more consistent with a car which will be manufactured by the hundreds of thousands. Concept vehicles have their place though – mostly to show off a company’s capabilities and satisfy egos. They just aren’t practical on scale. The truth is if Chevy produced at volume the concept version the average consumer wouldn’t be able to afford it. As a result, they round out the features, slap some plastic on it and Walla you’ve got a commuter vehicle fit for the masses at a price point which coincides with high volume sales. The medical device industry is no different. While industrial design is playing a much larger role today with device design, the fact of the matter is our industry struggles at times with over engineering products just like the auto industry. This can lead to a company losing sight of what the end user needs and wants, as well as if it can actually be manufactured to meet reimbursements. It’s for these reasons it’s important to consider design for manufacturing from the very beginning of the design process. If you do so, you stand to experience the following:
Considering DFM from the beginning of the design process is crucial for optimizing the manufacturing process, reducing costs, improving product quality, and ensuring a smoother path to market. It ultimately results in a more competitive and successful product with potential less risk downstream. The quickest way to overcome a business challenge is to get help from those who are experienced in besting your beast! The team at Square-1 Engineering is comprised of a variety of medical device technical and project management professionals who are subject matter experts in the areas of NPD, Quality, Compliance and Manufacturing Engineering. Learn more about how we can solve your work and project problems today to get you back on track!
Medtech Snapshot is back as we talk with Rohullah Latif who shares his insights on how artificial intelligence (AI) is changing the face of medical device manufacturing. Hear how AI is helping operations become more proactive, while reducing assembly errors. #artificialintelligence #AI #manufacturing #medtech #medicaldevice #snapshot The quickest way to overcome a business challenge is to get help from those who are experienced in besting your beast! The team at Square-1 Engineering is comprised of a variety of technical and project management professionals who are subject matter experts in the areas of NPD, Quality, Compliance and Manufacturing Engineering. Learn more about how we can solve your work and project problems today to get you back on track!
Sanjay Shrivastava, Ph.D. joins us on Medtech Snapshot to talk about medical device contract manufacturing partners - when to strategically engage them, especially if you are a startup, and the importance of "keeping the end in mind" throughout the process. Interested in catching up on our past Medtech Snapshot discussions? Visit #medicaldevice #medtech #snapshot #contractmanufacturing #contractmanufacturer #outsourcing #startup The quickest way to overcome a business challenge is to get help from those who are experienced in besting your beast! The team at Square-1 Engineering is comprised of a variety of technical and project management professionals who are subject matter experts in the areas of NPD, Quality, Compliance and Manufacturing Engineering. Learn more about how we can solve your work and project problems today to get you back on track!
Selecting the right medical device contract manufacturing partner is a critical decision that can significantly impact the success of your medical device development and production process. There are many advantages which come with working with a contract manufacturing partner, chief among them is it can potentially help you get out to the market quicker without having to go vertical, building up your own production system internally. The right partner can also offer cost savings as a result of their established supply chain partners, ability to scale and expertise in developing similar products. While those advantages can indeed be a blessing to a maturing business, landing the wrong partner can also be a curse. For this reason its vital device companies utilize a strategically planned process for how they are to approach and select a new contract manufacturing partner. The more due diligence one can do on the forefront the better the likelihood will be you will see success on the back end. Here are some important criteria to consider when evaluating potential contract manufacturing partners: 1. Experience and Expertise: Look for a partner with experience in manufacturing similar medical devices. Their expertise in the specific type of device, materials, processes, and technologies is vital to ensuring a smooth production process and high-quality output. 2. Capabilities and Facilities: Evaluate the contract manufacturer's facilities, equipment, and technological capabilities. Make sure they have the necessary infrastructure and resources to meet your manufacturing needs and scale as your project progresses. (HINT - learn about their employees. If they have high turnover with their internal staff this could create problems for you in the future) 3. Supply Chain Management: A robust supply chain is crucial for consistent component availability and cost-effectiveness. Assess the manufacturer's ability to manage the supply chain efficiently, including sourcing components, managing inventory, and handling potential disruptions. 4. Flexibility and Scalability: Consider the partner's ability to adapt to changes in project scope or production volume. A flexible partner can accommodate adjustments in design, production, and scheduling, which is important in the dynamic field of medical device development. MEDTECH SNAPSHOT: CONTRACT MANUFACTURING In this episode we are joined by Innova Vascular's CEO Sanjay Shiravastava who takes us through several pointers on when to consider finding a medical device contract manufacturing partner to aide in your go to market strategy. Here from Sanjay on why it's "important to begin with the end in mind". 5. Regulatory Compliance: Ensure that the contract manufacturer has a solid understanding of regulatory requirements, such as FDA (or equivalent) regulations, ISO standards (e.g., ISO 13485 for medical devices), and any other relevant local or international regulations. They should have a track record of successfully producing devices that meet these requirements. 6. Quality Management: A strong quality management system is crucial for medical device manufacturing. The partner should have established quality control processes, including inspection, testing, documentation, and corrective action procedures. They should also have a history of adhering to quality standards and maintaining good manufacturing practices. Their data and practices should be transparent and they should be open to you reviewing this information. 7. Geographical Location: The location of the contract manufacturer can impact shipping costs, lead times, and the ease of visiting the facility for inspections or meetings. 8. Intellectual Property Protection: Ensure that the contract manufacturer has protocols in place to protect your proprietary information and intellectual property throughout the manufacturing process. Do they work with a competitor of yours, if so how will they ensure your IP and product is protected? 9. References and Reputation: Research the manufacturer's reputation in the industry. Seek references from other companies they have worked with and review their track record to gauge their reliability, performance, and commitment to quality. 10. Cost and Pricing: While cost should not be the sole deciding factor, it's important to have a clear understanding of the pricing structure, including setup costs, unit costs, and any additional charges. Pricing should be clearly laid out and defined in your contract; stay away from partnerships where things seem murky and or inconsistent from a pricing perspective. 11. Long-Term Partnership Potential: Consider whether the contract manufacturer is interested in building a long-term partnership and is willing to support your future projects and iterations. 12. Employees and Training: Be mindful of how their employees are treated, trained and managed. High turnover at a contract manufacturer may mean you find yourself constantly involved int he training process to ensure the operators and assemblers working on your product are working as expected. The more turnover that occurs the more training that will need to take place, which means your partners training methods and consistently is critical to your success. High turnover and poor training will inevitably lead to yield problems. Before making a final decision, it's advisable to visit the contract manufacturer's facility, meet their team, and discuss your project in detail to ensure alignment and understanding. Taking the time to thoroughly assess potential partners will increase the likelihood of a successful and fruitful collaboration in medical device manufacturing. In this episode of Medtech Snapshot we discuss strategic supply chain strategies with medical device supply chain and manufacturing executive Jeff Brown. Key points in this discussion include addressing situations similar to China and Taiwan, solutions to hedge yourself against such global challenges, including but not limited to supplier risk, back-up capacity and vertical integration. Need help sorting through your supply chain and or contract manufacturing challenges? Learn more about Square-1 Engineering's capabilities as it relates to all things manufacturing engineering: NPI, tech transfer, pilot manufacturing, process development and improvement, supply chain management, etc. Click HERE to learn more.
Companies which design, develop and manufacture products often times need additional support to accomplish their goals. These are the three main reasons companies use outsourced engineering service firms. About the AuthorTravis Smith is the founder and managing director of Square-1 Engineering, a medical device consulting firm, providing end to end engineering and compliance services. He successfully served the life sciences marketplace in SoCal for over 15 years and has been recognized as a ‘40 Under 40’ honoree by the Greater Irvine Chamber of Commerce as a top leader in Orange County, CA. Categories
All
Archives
April 2024
|
Visit Square-1's
|
|







 RSS Feed
RSS Feed


