|
Here’s a challenge I see quite often while we’re on a client project involving mechanical design or CAD work. Does this familiar? Someone is tasked with designing a new sub-assembly or component for an existing product. As they get underway their work on face value gets the company to a conclusion where the design/ drawing is technically complete. As such, this person is able to check the proverbial box for ‘task completed’ and move on to the next assignment. While the work may have technically been completed, it often is done in a fashion which causes all sorts of problems down the road for the company, including other employees working on the same project within the same organization as well as their external suppliers. How is it someone can complete a design project satisfactory on the surface yet problems arise down the road with that very same design, which had been previously approved? Answer: the devil is in the details, or lack thereof, to be more specific. The reason why companies and or their respective employees experience this is because they aren’t following a formal and documented ‘gold standard’ for their product design practices. Simply put, they lack discipline with design fundamentals. As a result of a lack of design standards (and perhaps training) employees are left to decide for themselves how to complete a task which may get them to the finish line but the approach, process and details along the way can have wild variances and interpretations. While this may be commonplace and old news to many of you reading this article, the reality is the actual practice of designing a product with repeatable ‘gold standards’ is anything but common sense or consistent in the workplace. When our approach to design is fast and loose we experience the following:
When these issues show up it causes companies to reinvest dollars and resources into their work in order to move the project forward to get it to a point of where it can be properly advanced along the product development life cycle. This reinvestment is unnecessary and a huge time suck. We see this a lot when a medical device OEM has a contract manufacturer (CM) do some of their design work. In more times than I can count the work which is produced in this scenario is rough, limited with detail and documentation, almost never parametrically driven, and close to useless in other scenarios. Don’t fall for the trap of “we just need drawings.” While that may be the case in the moment, this will almost always cause you more work and funds down the road. For these reasons it’s vital companies implement a ‘gold standard’ in their design work which their employees and suppliers follow to ensure the work each party is facilitating makes it to the finish line in the same format, intent and approach. This unification of process increases the likelihood design work is done correctly while also ensuring future usage of said designs doesn’t require additional unnecessary iterations or complete redesigns. If implementing a ‘gold standard’ for your design and product development practices could be a benefit to your team or company, here are some of the key points to consider:
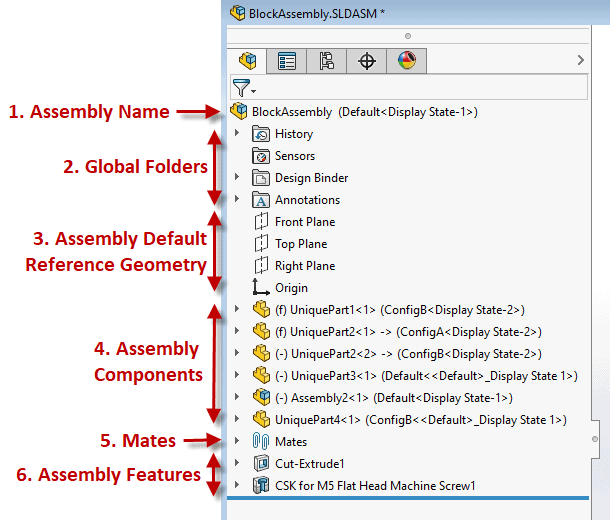
Example below: A well laid out Solidworks Assembly Feature Manager Design Tree If you, and or your company, lacks a ‘gold standard’ for your product design efforts you are inevitably wasting time and resources. This also has a direct correlation to a suppliers’ ability to help with outsourced work causing the overall project to be more challenging and lengthier than necessary (prototyping, manufacturing, etc.) While this isn’t a fun realization there is hope! Here’s how to fix it. Start right away by developing a best practice plan. This will help you and your team form an outline for what design practices and approaches are ideal for your product and technology, which aren’t, etc. From there setup a review plan to provide feedback on all work performed. Once the infrastructure of your new gold standard system is established you’ll want to asses the skills of your team and develop a training program which can be offered to both new and existing employees.
0 Comments
Sanjay Shrivastava, Ph.D. joins us on Medtech Snapshot to talk about medical device contract manufacturing partners - when to strategically engage them, especially if you are a startup, and the importance of "keeping the end in mind" throughout the process. Interested in catching up on our past Medtech Snapshot discussions? Visit #medicaldevice #medtech #snapshot #contractmanufacturing #contractmanufacturer #outsourcing #startup The quickest way to overcome a business challenge is to get help from those who are experienced in besting your beast! The team at Square-1 Engineering is comprised of a variety of technical and project management professionals who are subject matter experts in the areas of NPD, Quality, Compliance and Manufacturing Engineering. Learn more about how we can solve your work and project problems today to get you back on track!
Selecting the right medical device contract manufacturing partner is a critical decision that can significantly impact the success of your medical device development and production process. There are many advantages which come with working with a contract manufacturing partner, chief among them is it can potentially help you get out to the market quicker without having to go vertical, building up your own production system internally. The right partner can also offer cost savings as a result of their established supply chain partners, ability to scale and expertise in developing similar products. While those advantages can indeed be a blessing to a maturing business, landing the wrong partner can also be a curse. For this reason its vital device companies utilize a strategically planned process for how they are to approach and select a new contract manufacturing partner. The more due diligence one can do on the forefront the better the likelihood will be you will see success on the back end. Here are some important criteria to consider when evaluating potential contract manufacturing partners: 1. Experience and Expertise: Look for a partner with experience in manufacturing similar medical devices. Their expertise in the specific type of device, materials, processes, and technologies is vital to ensuring a smooth production process and high-quality output. 2. Capabilities and Facilities: Evaluate the contract manufacturer's facilities, equipment, and technological capabilities. Make sure they have the necessary infrastructure and resources to meet your manufacturing needs and scale as your project progresses. (HINT - learn about their employees. If they have high turnover with their internal staff this could create problems for you in the future) 3. Supply Chain Management: A robust supply chain is crucial for consistent component availability and cost-effectiveness. Assess the manufacturer's ability to manage the supply chain efficiently, including sourcing components, managing inventory, and handling potential disruptions. 4. Flexibility and Scalability: Consider the partner's ability to adapt to changes in project scope or production volume. A flexible partner can accommodate adjustments in design, production, and scheduling, which is important in the dynamic field of medical device development. MEDTECH SNAPSHOT: CONTRACT MANUFACTURING In this episode we are joined by Innova Vascular's CEO Sanjay Shiravastava who takes us through several pointers on when to consider finding a medical device contract manufacturing partner to aide in your go to market strategy. Here from Sanjay on why it's "important to begin with the end in mind". 5. Regulatory Compliance: Ensure that the contract manufacturer has a solid understanding of regulatory requirements, such as FDA (or equivalent) regulations, ISO standards (e.g., ISO 13485 for medical devices), and any other relevant local or international regulations. They should have a track record of successfully producing devices that meet these requirements. 6. Quality Management: A strong quality management system is crucial for medical device manufacturing. The partner should have established quality control processes, including inspection, testing, documentation, and corrective action procedures. They should also have a history of adhering to quality standards and maintaining good manufacturing practices. Their data and practices should be transparent and they should be open to you reviewing this information. 7. Geographical Location: The location of the contract manufacturer can impact shipping costs, lead times, and the ease of visiting the facility for inspections or meetings. 8. Intellectual Property Protection: Ensure that the contract manufacturer has protocols in place to protect your proprietary information and intellectual property throughout the manufacturing process. Do they work with a competitor of yours, if so how will they ensure your IP and product is protected? 9. References and Reputation: Research the manufacturer's reputation in the industry. Seek references from other companies they have worked with and review their track record to gauge their reliability, performance, and commitment to quality. 10. Cost and Pricing: While cost should not be the sole deciding factor, it's important to have a clear understanding of the pricing structure, including setup costs, unit costs, and any additional charges. Pricing should be clearly laid out and defined in your contract; stay away from partnerships where things seem murky and or inconsistent from a pricing perspective. 11. Long-Term Partnership Potential: Consider whether the contract manufacturer is interested in building a long-term partnership and is willing to support your future projects and iterations. 12. Employees and Training: Be mindful of how their employees are treated, trained and managed. High turnover at a contract manufacturer may mean you find yourself constantly involved int he training process to ensure the operators and assemblers working on your product are working as expected. The more turnover that occurs the more training that will need to take place, which means your partners training methods and consistently is critical to your success. High turnover and poor training will inevitably lead to yield problems. Before making a final decision, it's advisable to visit the contract manufacturer's facility, meet their team, and discuss your project in detail to ensure alignment and understanding. Taking the time to thoroughly assess potential partners will increase the likelihood of a successful and fruitful collaboration in medical device manufacturing. In this episode of Medtech Snapshot we discuss strategic supply chain strategies with medical device supply chain and manufacturing executive Jeff Brown. Key points in this discussion include addressing situations similar to China and Taiwan, solutions to hedge yourself against such global challenges, including but not limited to supplier risk, back-up capacity and vertical integration. Need help sorting through your supply chain and or contract manufacturing challenges? Learn more about Square-1 Engineering's capabilities as it relates to all things manufacturing engineering: NPI, tech transfer, pilot manufacturing, process development and improvement, supply chain management, etc. Click HERE to learn more.
About the AuthorTravis Smith is the founder and managing director of Square-1 Engineering, a medical device consulting firm, providing end to end engineering and compliance services. He successfully served the life sciences marketplace in SoCal for over 15 years and has been recognized as a ‘40 Under 40’ honoree by the Greater Irvine Chamber of Commerce as a top leader in Orange County, CA. Categories
All
Archives
July 2024
|
Visit Square-1's
|
|




 RSS Feed
RSS Feed


