|
This 3-part series of Medtech Snapshot features Jeff Gable, medtech embedded software SME. In part-3 Jeff dives further into strategies for developing design requirements and how staying away from 'TBD requirement' data is advisable.
Hear how flushing out requirements to their full level of detail is vital, while establishing appropriate traceability processes in tools like Matrix or Jama will keep you organized and moving your team and project forward. #agile #embeddedsoftware #productdevelopment #medicaldevice #medtech #NPD #FDA #designcontrol #designinput #snapshot #requirements
0 Comments
Medtech Snapshot - Agile and NPD episode part-2 of 3 features Jeff Gable, embedded software SME, diving in on Agile and NPD activities.
Jeff walks us through his strategy for Agile development with FDA design controls, specifically within the design input phase. Hear the first two steps of his four step approach. #agile #embeddedsoftware #productdevelopment #medicaldevice #medtech #NPD #FDA #designcontrol #designinput #snapshot Ep 31 of Medtech Snapshot features Krystal Santiago sharing with us strategies for working with regulatory agencies during a submission and how utilizing an intermediary can be beneficial. This impacts decision making with clinical studies, product codes and requirements, pre-submission planning, etc.
Did you miss the last 30 episodes of Medtech Snapshot? If so, check out our archives at https://www.sqr1services.com/white-papers/category/snapshot #medtechsnapshot #snapshot #medtech #medicaldevice #FDA #regulatory #productsubmission #compliance #510k #PMA In this episode of Medtech Snapshot we're joined by Philippe JEAN who walks us through strategies for medical device supply chain management, in particular dual sourcing for critical components. Want more Medtech Snapshot? Check out our archives featuring dozens of episodes covering medtech content from NPD, Quality, Clinical, Ops and Manuf Engineering HERE #supplychain #sourcing #logistics #supplychainmanagement #strategy #medtech #medicaldevice #snapshot Medtech Snapshot Part 2:2 continues the discussion as Trisha Aure covers considerations when transitioning from 21 CFR 820 to ISO 13485.
Hear how due diligence and training play a crucial role in the transition process and the impact it has on our employees. #iso13485 #regulation #compliance #fda #21CFR #riskmanagement #medicaldevice #medtech #news #podcast #snapshot #regulatoryaffairs In this 2-part series of Medtech Snapshot we're joined by Square-1 Engineering Director of Delivery & Operations Trisha Aure as she walks us through the highlights of our article 'FDA Announcement: 21 CFR 820 and ISO 13485 Guidance'
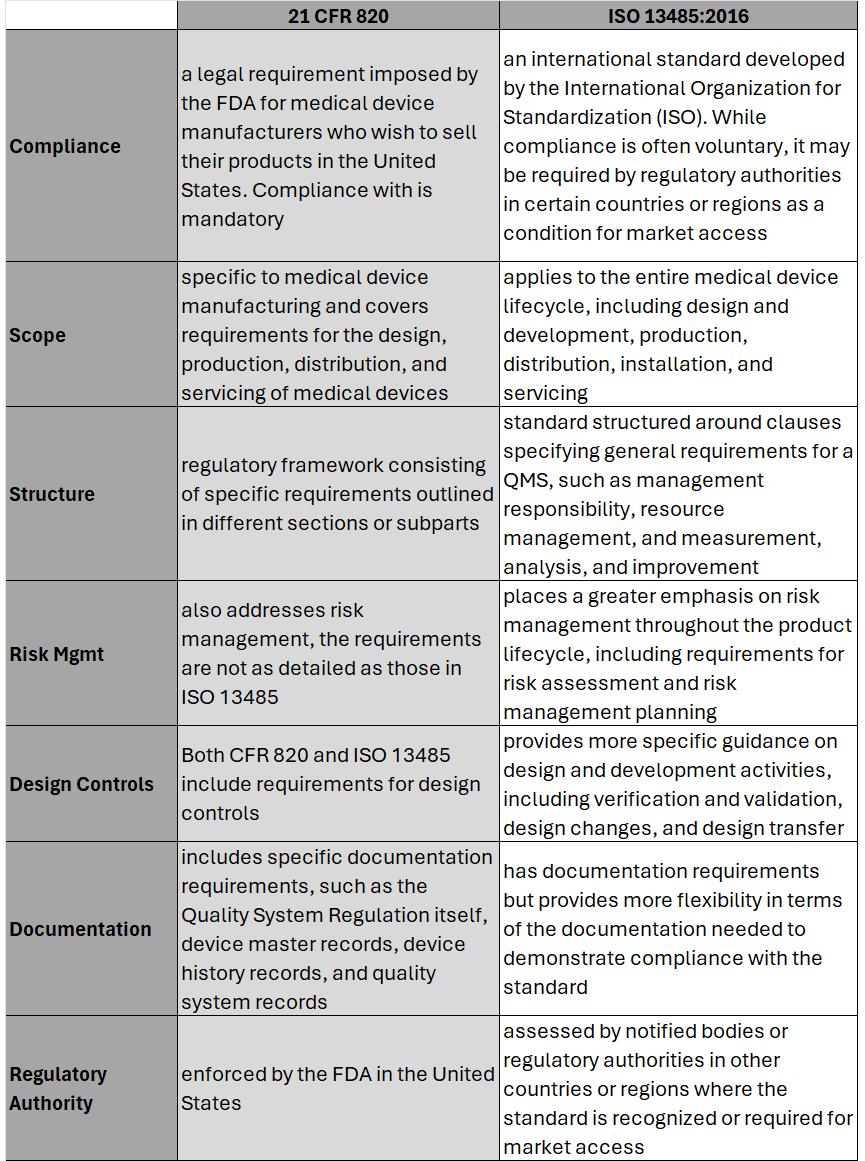
Hear the areas where 21 CFR 820 differs most from ISO 13485 and what this means for medical device OEMs. Part-2 of this series will cover the process to transition and key considerations when doing so. #iso13485 #regulation #compliance #fda #21CFR #riskmanagement #medicaldevice #medtech #news #podcast #snapshot #regulatoryaffairs This episode of Medtech Snapshot features our good friend Jeff Gable, medtech embedded software SME, diving in on Agile and NPD activities. Jeff walks us through his strategy for Agile development with FDA design controls, specifically within the design input phase. Hear Jeff's 4 step approach and how a tight feedback loop is critical between requirements and system design/ architectures. #agile #embeddedsoftware #productdevelopment #medicaldevice #medtech #NPD #FDA #designcontrol #designinput #snapshot On February 2nd, 2024 the U.S. Food and Drug Administration (FDA) made its formal announcement and ruling providing guidance on 21 CFR Part 820 which up to that point was the standard overseeing medical device quality system regulation and current good manufacturing practices (cGMP) in the United States of America. The big news – 21 CFR 820 will be heavily amended to incorporate ISO 13485 as the leading guidance for Quality Management System Regulation (QMSR) and cGMP. While the news wasn’t a surprise, it does put a final note on the direction the agency intends to take for medical device practices moving forward, especially as it relates to risk management. While many device OEMs already utilize ISO as their leading regulation standard, those who don’t will have two years to adjust to these changes to be in compliance effective February 2nd, 2026. Here’s what you need to know as it relates to the differences between 21 CFR 820 and ISO 13485, as well as considerations OEMs should take into account in order to meet the 2026 deadline. Big Picture:
Key Differences: Transitioning to ISO 13485 Transitioning from CFR 820 to ISO 13485 involves several steps to ensure compliance with the ISO standard. A general outline of the steps an OEM should take to transition may include: 1. Understand the Requirements of ISO 13485: Familiarize yourself with the requirements of ISO 13485. This includes understanding the structure of the standard, its key clauses, and any specific requirements which may differ from CFR 820. (see above table for highlights) 2. Gap Analysis: Conduct a thorough gap analysis to identify the differences between your current quality management system under CFR 820 and the requirements of ISO 13485. This will help you determine what changes, if any, need to be made to your existing processes, procedures, and documentation. This is also a great time to do a review of your QMS tool to determine if it is an appropriate tool for future use. 3. Document Review and Update: Review your existing documentation, including quality manuals, procedures, work instructions, and forms, to ensure they align with the requirements of ISO 13485. Update or create new documents as necessary to meet the standard's requirements. 4. Training and Awareness: Provide training to relevant personnel to ensure they understand the requirements of ISO 13485 and their roles in implementing and maintaining the QMS. This may include training on new procedures, processes, and documentation. 5. Implementation of New Processes: Implement any new processes or procedures required by ISO 13485. This may include processes related to risk management, design and development, purchasing, production, and service provisions. 6. Internal Audits: Conduct internal audits of your QMS to verify compliance with ISO 13485 requirements. Identify any non-conformities and take corrective actions to address them. 7. Management Review: Hold management reviews to evaluate the effectiveness of the QMS and identify opportunities for improvement. Ensure top management is actively involved in the transition process and committed to maintaining the QMS. 8. Certification Audit: BEFORE you consider this step be sure to speak with a regulatory affairs subject matter expert to ensure it is necessary. Once you believe your QMS is fully compliant with ISO 13485, engage a certification body to conduct a certification audit. The audit will assess your organization's compliance with the standard and determine if you are eligible for certification. 9. Address Non-conformities: If any non-conformities are identified during the certification audit, take corrective actions to address them. The certification body will typically require verification that corrective actions have been implemented before issuing the ISO 13485 certificate. 10. Continual Improvement: Continuously monitor and improve your QMS to ensure ongoing compliance with ISO 13485 and to enhance the efficiency and effectiveness of your processes. Although the 21 CFR 820 and ISO 13485 vary in their structure, and at times use different terminology to describe similar concepts, 21 CFR 820 and ISO 13485 are substantially similar in that both prioritize principles such as risk management, design controls, and continual process improvement. It’s possible as organizations begin to look at their current standards and systems, they will find the transition process is not as cumbersome as initially thought. While this is an obvious assumption, it’s important to note regulatory affairs professionals should be counseled throughout this entire process to ensure appropriateness of adoption and change management. Here’s a challenge I see quite often while we’re on a client project involving mechanical design or CAD work. Does this familiar? Someone is tasked with designing a new sub-assembly or component for an existing product. As they get underway their work on face value gets the company to a conclusion where the design/ drawing is technically complete. As such, this person is able to check the proverbial box for ‘task completed’ and move on to the next assignment. While the work may have technically been completed, it often is done in a fashion which causes all sorts of problems down the road for the company, including other employees working on the same project within the same organization as well as their external suppliers. How is it someone can complete a design project satisfactory on the surface yet problems arise down the road with that very same design, which had been previously approved? Answer: the devil is in the details, or lack thereof, to be more specific. The reason why companies and or their respective employees experience this is because they aren’t following a formal and documented ‘gold standard’ for their product design practices. Simply put, they lack discipline with design fundamentals. As a result of a lack of design standards (and perhaps training) employees are left to decide for themselves how to complete a task which may get them to the finish line but the approach, process and details along the way can have wild variances and interpretations. While this may be commonplace and old news to many of you reading this article, the reality is the actual practice of designing a product with repeatable ‘gold standards’ is anything but common sense or consistent in the workplace. When our approach to design is fast and loose we experience the following:
When these issues show up it causes companies to reinvest dollars and resources into their work in order to move the project forward to get it to a point of where it can be properly advanced along the product development life cycle. This reinvestment is unnecessary and a huge time suck. We see this a lot when a medical device OEM has a contract manufacturer (CM) do some of their design work. In more times than I can count the work which is produced in this scenario is rough, limited with detail and documentation, almost never parametrically driven, and close to useless in other scenarios. Don’t fall for the trap of “we just need drawings.” While that may be the case in the moment, this will almost always cause you more work and funds down the road. For these reasons it’s vital companies implement a ‘gold standard’ in their design work which their employees and suppliers follow to ensure the work each party is facilitating makes it to the finish line in the same format, intent and approach. This unification of process increases the likelihood design work is done correctly while also ensuring future usage of said designs doesn’t require additional unnecessary iterations or complete redesigns. If implementing a ‘gold standard’ for your design and product development practices could be a benefit to your team or company, here are some of the key points to consider:
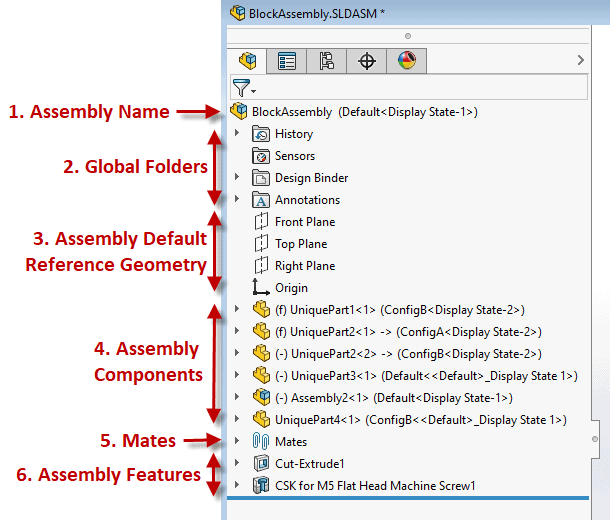
Example below: A well laid out Solidworks Assembly Feature Manager Design Tree If you, and or your company, lacks a ‘gold standard’ for your product design efforts you are inevitably wasting time and resources. This also has a direct correlation to a suppliers’ ability to help with outsourced work causing the overall project to be more challenging and lengthier than necessary (prototyping, manufacturing, etc.) While this isn’t a fun realization there is hope! Here’s how to fix it. Start right away by developing a best practice plan. This will help you and your team form an outline for what design practices and approaches are ideal for your product and technology, which aren’t, etc. From there setup a review plan to provide feedback on all work performed. Once the infrastructure of your new gold standard system is established you’ll want to asses the skills of your team and develop a training program which can be offered to both new and existing employees. Medtech Snapshot: Fundraising and the Sales Funnel with Scott Nelson, CEO Fastwave Medical1/15/2024 Medtech Snapshot episode #22 features Fastwave Medical's CEO Scott Nelson sharing insights on the impact of using the 'sales funnel' process for medical device fundraising purposes. Listen how Scott's approach to fundraising starts off by casting a wide net via branding and mission/ story telling efforts then intentionally narrows the funding field to a smaller audience of potential investors which are aligned with his company's mission. Want more info? Check out Square-1's other Medtech Snapshot episodes at https://www.sqr1services.com/white-papers/category/snapshot Learn about Fastwave Medical's mission and product offering at https://www.fastwavemedical.com/ The quickest way to overcome a business challenge is to get help from those who are experienced in besting your beast! The team at Square-1 Engineering is comprised of a variety of technical and project management professionals who are subject matter experts in the areas of NPD, Quality, Compliance and Manufacturing Engineering.
Learn more about how we can solve your work and project problems today to get you back on track! VISIT HERE About the AuthorTravis Smith is the founder and managing director of Square-1 Engineering, a medical device consulting firm, providing end to end engineering and compliance services. He successfully served the life sciences marketplace in SoCal for over 15 years and has been recognized as a ‘40 Under 40’ honoree by the Greater Irvine Chamber of Commerce as a top leader in Orange County, CA. Categories
All
Archives
April 2024
|
Visit Square-1's
|
|





 RSS Feed
RSS Feed


