|
This 3-part series of Medtech Snapshot features Jeff Gable, medtech embedded software SME. In part-3 Jeff dives further into strategies for developing design requirements and how staying away from 'TBD requirement' data is advisable.
Hear how flushing out requirements to their full level of detail is vital, while establishing appropriate traceability processes in tools like Matrix or Jama will keep you organized and moving your team and project forward. #agile #embeddedsoftware #productdevelopment #medicaldevice #medtech #NPD #FDA #designcontrol #designinput #snapshot #requirements
0 Comments
Medtech Snapshot - Agile and NPD episode part-2 of 3 features Jeff Gable, embedded software SME, diving in on Agile and NPD activities.
Jeff walks us through his strategy for Agile development with FDA design controls, specifically within the design input phase. Hear the first two steps of his four step approach. #agile #embeddedsoftware #productdevelopment #medicaldevice #medtech #NPD #FDA #designcontrol #designinput #snapshot This episode of Medtech Snapshot features our good friend Jeff Gable, medtech embedded software SME, diving in on Agile and NPD activities. Jeff walks us through his strategy for Agile development with FDA design controls, specifically within the design input phase. Hear Jeff's 4 step approach and how a tight feedback loop is critical between requirements and system design/ architectures. #agile #embeddedsoftware #productdevelopment #medicaldevice #medtech #NPD #FDA #designcontrol #designinput #snapshot Here’s a challenge I see quite often while we’re on a client project involving mechanical design or CAD work. Does this familiar? Someone is tasked with designing a new sub-assembly or component for an existing product. As they get underway their work on face value gets the company to a conclusion where the design/ drawing is technically complete. As such, this person is able to check the proverbial box for ‘task completed’ and move on to the next assignment. While the work may have technically been completed, it often is done in a fashion which causes all sorts of problems down the road for the company, including other employees working on the same project within the same organization as well as their external suppliers. How is it someone can complete a design project satisfactory on the surface yet problems arise down the road with that very same design, which had been previously approved? Answer: the devil is in the details, or lack thereof, to be more specific. The reason why companies and or their respective employees experience this is because they aren’t following a formal and documented ‘gold standard’ for their product design practices. Simply put, they lack discipline with design fundamentals. As a result of a lack of design standards (and perhaps training) employees are left to decide for themselves how to complete a task which may get them to the finish line but the approach, process and details along the way can have wild variances and interpretations. While this may be commonplace and old news to many of you reading this article, the reality is the actual practice of designing a product with repeatable ‘gold standards’ is anything but common sense or consistent in the workplace. When our approach to design is fast and loose we experience the following:
When these issues show up it causes companies to reinvest dollars and resources into their work in order to move the project forward to get it to a point of where it can be properly advanced along the product development life cycle. This reinvestment is unnecessary and a huge time suck. We see this a lot when a medical device OEM has a contract manufacturer (CM) do some of their design work. In more times than I can count the work which is produced in this scenario is rough, limited with detail and documentation, almost never parametrically driven, and close to useless in other scenarios. Don’t fall for the trap of “we just need drawings.” While that may be the case in the moment, this will almost always cause you more work and funds down the road. For these reasons it’s vital companies implement a ‘gold standard’ in their design work which their employees and suppliers follow to ensure the work each party is facilitating makes it to the finish line in the same format, intent and approach. This unification of process increases the likelihood design work is done correctly while also ensuring future usage of said designs doesn’t require additional unnecessary iterations or complete redesigns. If implementing a ‘gold standard’ for your design and product development practices could be a benefit to your team or company, here are some of the key points to consider:
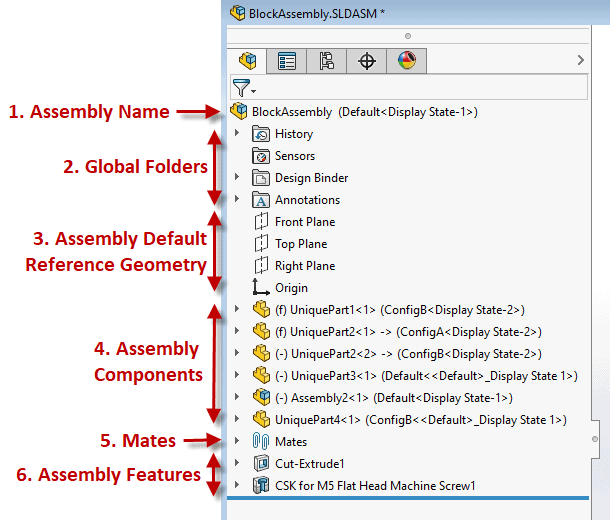
Example below: A well laid out Solidworks Assembly Feature Manager Design Tree If you, and or your company, lacks a ‘gold standard’ for your product design efforts you are inevitably wasting time and resources. This also has a direct correlation to a suppliers’ ability to help with outsourced work causing the overall project to be more challenging and lengthier than necessary (prototyping, manufacturing, etc.) While this isn’t a fun realization there is hope! Here’s how to fix it. Start right away by developing a best practice plan. This will help you and your team form an outline for what design practices and approaches are ideal for your product and technology, which aren’t, etc. From there setup a review plan to provide feedback on all work performed. Once the infrastructure of your new gold standard system is established you’ll want to asses the skills of your team and develop a training program which can be offered to both new and existing employees. Medtech Snapshot: Forward Progress in Product Development with Glen Rabito, COO of Nidus Biomedical1/10/2024 Medtech Snapshot episode #21 features Nidus Biomedical COO Glen Rabito talking through the five considerations to keep product development moving forward, especially in the early phases of development.
#medtech #snapshot #medicaldevice #productdevelopment #NPD #riskmanagement #userneeds #patientsafety Medical device companies play a critical role in advancing healthcare as their ability to diagnose, monitor, and treat medical conditions allow patients like you and I the opportunity to recover and live longer. Device companies carry a heavy burden on our behalf and that burden starts with product risk. One of the biggest challenges an OEM medical device organization will be faced with is managing risk, especially during the early stages of product development. The integration of risk management into design control (ISO 14971) is essential for identifying, assessing, and mitigating potential risks associated with the design and development of a medical device. Given risk management is a part of nearly every development process, and is a primary focus of all regulatory agencies like the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA), why is it then so many medical device companies struggle with sound risk management strategies? The failure to address risks adequately can lead to a whole host of problems ranging from regulatory non-compliance, compromised patient safety, financial setbacks, and in severe situations criminal prosecution of executives. Needless to say, understanding why medical device companies come up short with their risk management strategy and how you can avoid that for yourself is key to your future success. In this article, we will explore some of the key reasons behind risk management failure. Most Common Risk Failure Factor - Inadequate Understanding of Regulatory Requirements: One of the primary reasons for failure in risk management is an insufficient understanding of the complex and evolving regulatory landscape. Medical device companies must navigate a web of regulations, standards, and guidelines to ensure compliance. Failing to keep abreast of these requirements can result in flawed risk assessments, inadequate risk mitigation measures, and ultimately, regulatory sanctions. Poor Integration of Risk Management into Product Development: Successful risk management should be an integral part of the product development lifecycle. However, some companies make the mistake of treating it as a standalone process rather than integrating it seamlessly into every stage of development. When risk management is an afterthought, essential risks may be overlooked, leading to suboptimal product designs, increased failure rates, and compromised patient outcomes. Lack of Cross-Functional Collaboration: Effective risk management requires collaboration across various departments, including research and development, regulatory affairs, quality assurance, and manufacturing. Failure to establish clear communication channels and encourage cross-functional collaboration can result in siloed decision-making. This lack of coordination can lead to critical risks being underestimated or missed entirely. Insufficient Resources and Expertise: Some medical device companies fail in risk management due to resource constraints and a shortage of expertise. This can manifest in inadequate training for personnel responsible for risk management, insufficient allocation of time and budget, and a lack of access to external expertise. Without the necessary resources, companies may struggle to conduct comprehensive risk assessments and implement effective risk mitigation strategies. Overemphasis on Short-Term Goals: Pressure to meet short-term financial goals can sometimes lead companies to prioritize speed to market over thorough risk analysis. This can result in hasty decision-making and inadequate risk identification and mitigation. Companies need to strike a balance between meeting market demands and ensuring the safety and efficacy of their medical devices in the long run. Failure to Learn from Industry Incidents: The medical device industry has witnessed several high-profile incidents related to product failures and patient harm. Failure to learn from these incidents and implement lessons learned into future risk management strategies can perpetuate the same mistakes. Companies must actively analyze industry incidents, update risk management processes accordingly, and continuously improve their practices. Ineffective Communication with Stakeholders: Communication is crucial in risk management, both internally and externally. Companies that fail to communicate effectively with their stakeholders, including regulatory bodies, healthcare professionals, and patients, may face increased scrutiny and regulatory challenges. Transparency and open communication are essential for building trust and demonstrating commitment to patient safety. In the highly regulated and dynamic field of medical devices, effective risk management is not just a regulatory requirement - it is a fundamental aspect of ensuring patient safety and the success of a company. Understanding the pitfalls that lead to failures in risk management can help medical device companies proactively address these challenges. By prioritizing compliance, integrating risk management into every stage of product development, fostering cross-functional collaboration, and learning from industry incidents, companies can enhance their risk management strategies and contribute to the advancement of healthcare with safe and reliable medical devices. The quickest way to overcome a business challenge is to get help from those who are experienced in besting your beast! The team at Square-1 Engineering is comprised of a variety of technical and project management professionals who are subject matter experts in the areas of NPD, Quality, Compliance and Manufacturing Engineering. Learn more about how we can solve your work and project problems today to get you back on track!
Our most recent Medtech Snapshot features product development executive Barry Fulkerson as he takes us through his strategy of obtaining user needs data on the cheap and how creating a patient advisory board can help get you there.
We have archives! To watch and listen in to our past Medtech Snapshot episodes visit https://www.sqr1services.com/white-papers/category/snapshot #userneeds #medtech #snapshot #medicaldevice #productdevelopment #conceptdevelopment #patient At the forefront of the medical device new product development (NPD) process we typically find a list of considerations we’re trying to address within our product all while coming up with a solution which meets marketplace needs. Talk to anyone in R&D, or downstream marketing for that matter, and you’ll commonly hear the list of ‘needs’ for a new product can seem never-ending. What you may find surprising is how often design for manufacturability (DFM) is not considered in the beginning of the development process. DFM is an important, and often overlooked, part of product development which involves designing a product in a way which makes it easier and more cost-effective to manufacture commercially. While embedding DFM into the process early seems like a logical part of the process, what plays out in reality is an NPD process that is focused on creativity and validating a technology yet can lack appreciation for the future state of the product and it’s foray into the market. So why is it companies may overlook incorporating DFM into the development process? For starters, many of us who design products on the front end, have little perspective, or perhaps no perspective on what it takes to actually produce a scalable product on the back end. Unless you’ve worked on a manufacturing floor or you’ve walked through an entire new product introduction (NPI) cycle, it’s easy to misunderstand all which goes into producing a product to bring to market. The design process also requires creativity and ingenuity in order to find new approaches, break common perceptions of technology capabilities, etc. It’s about ideation! Unfortunately thinking down the road about how it will be built for commercial purposes is “someone else’s problem”. To illustrate this point let’s look towards the automotive industry at a product we all know, which also happens to be a heavily regulated industry, like the medical device industry. If you have ever been to a large-scale automotive show, like the LA Auto Show in Los Angeles, CA, what you’ll see are concept cars on display to wow the eyes and entice the senses. Ironically, if those same concept cars ever got to production, you’ll find their characteristics which made the vehicle look incredible are often diluted down to features and benefits which consumers will accept and are cost efficient to produce. Case in point, the two images below shows a 2010 Chevy Volt electric vehicle. The top image is the concept vehicle – sleek, sophisticated, sharp lines, aggressive character and eye catching. Almost resembles a sports car. The bottom image is the production vehicle – commuter extraordinaire with features which are dull, less angular and more consistent with a car which will be manufactured by the hundreds of thousands. Concept vehicles have their place though – mostly to show off a company’s capabilities and satisfy egos. They just aren’t practical on scale. The truth is if Chevy produced at volume the concept version the average consumer wouldn’t be able to afford it. As a result, they round out the features, slap some plastic on it and Walla you’ve got a commuter vehicle fit for the masses at a price point which coincides with high volume sales. The medical device industry is no different. While industrial design is playing a much larger role today with device design, the fact of the matter is our industry struggles at times with over engineering products just like the auto industry. This can lead to a company losing sight of what the end user needs and wants, as well as if it can actually be manufactured to meet reimbursements. It’s for these reasons it’s important to consider design for manufacturing from the very beginning of the design process. If you do so, you stand to experience the following:
Considering DFM from the beginning of the design process is crucial for optimizing the manufacturing process, reducing costs, improving product quality, and ensuring a smoother path to market. It ultimately results in a more competitive and successful product with potential less risk downstream. The quickest way to overcome a business challenge is to get help from those who are experienced in besting your beast! The team at Square-1 Engineering is comprised of a variety of medical device technical and project management professionals who are subject matter experts in the areas of NPD, Quality, Compliance and Manufacturing Engineering. Learn more about how we can solve your work and project problems today to get you back on track!
When you’re early in the development process it can seem like there’s a never ending list of activities and projects your R&D team needs to take on. When you’re in this mode its vital R&D leaders assess time, resources and associated risks with the product they’re developing. Medical device product development executive Arif Iftekhar walks us through how to focus your team and address the most important product risks head on at an early stage. Recently our company published a poll online offering up the following question for the medical device community: “What is the most important factor to consider when developing a medical device product?” At the close of the poll dozens of people had cast their votes for what they believed to be the factors affecting product development the most. The poll options included:
If you have been in industry for any length of time you know there are dozens of factors which can and often have a direct impact on the product development process. While there are dozens to consider, such as PRS (Product Requirement Specification), planning, user experience, DFM, etc. what we know to be true is each of these factors carry varying weights of impact. They are not all equal in measure or influence. As our poll launched and picked up steam one of the four factors listed as an option began to take a commanding lead. The respondents, who are largely made up of medical device professionals and executives, had identified a common factor which stood above the rest in its ability to impact positively or negatively the product development process. What was this most important factor? Would you have guessed ‘Having the Right Team in Place’ is the number one factor which determines success when developing a medical device product? ‘Having the Right Team in Place’ was identified by 51% of the respondents as being the most important factor which directly contributes to the success of medical device product development. The other options broke down as follows: Simply put – having the right team in place covers all of the other areas that potentially could produce challenges during the product development cycle. Whereas the inverse is certainly all too true. When we have the wrong team in place, or teammates lacking the capabilities to facilitate their job as needed by the company, inevitably problems go arise which hold back otherwise good opportunities and technology offerings. Jim Collins, celebrated author (books like ‘Good to Great’ & ‘Built to Last’) and business management guru, is quoted as saying “Leaders of great companies start by getting the right people on the bus, the wrong people off the bus, and the right people in the right seats.” What this means is it’s more about the people than it is the technology or problem you’re solving. This is an important lesson, especially for first time entrepreneurs and startup executives. You can have the best product idea in the world, one that is in high demand, but if you don’t have the right team in place you’ll most likely spin your wheels while blowing out copious amounts of money in the process. We’ve also seen this reality in person dozens of times. As a medical device consulting firm we work with a lot of companies, both start up and conglomerate alike. One of the consistent characteristics we see within the companies which are able to drive success, often times repeated success, is their management team is comprised of experts in their particular field who know how to both lead and operate in the weeds. They both strategic and tactical, able to plan for the long term while addressing todays shorter term needs. As a result, they know how the job is done and therefore can either lead or delegate those tasks helping to guide their department or team to successful completion. When you have the right people on the team (your bus) you will then find opportunities (the medical problem you’ll solve) to move forward with. Following this process you’ll also have a far better chance of facilitating that opportunity through the development process and into commercialization, or acquisition. About the AuthorTravis Smith is the founder and managing director of Square-1 Engineering, a medical device consulting firm, providing end to end engineering and compliance services. He successfully served the life sciences marketplace in SoCal for over 15 years and has been recognized as a ‘40 Under 40’ honoree by the Greater Irvine Chamber of Commerce as a top leader in Orange County, CA. Categories
All
Archives
July 2024
|
Visit Square-1's
|
|








 RSS Feed
RSS Feed


