|
One of the biggest challenges professionals face when starting a new job is how they navigate ingratiating themselves into the new company and culture they’re now surrounded by. No two companies are alike, which also means practices, processes and procedures can vary wildly from one company to another. How a new employee, including new management, sorts through this can make or break their ability to be received well by their fellow colleagues while having a good career at their new employer. Imagine you’re in your second week of employment and you begin to uncover a variety of compliance issues like a lack of regulatory understanding throughout the company, poor or missing documentation, insufficient training, little to no post-market surveillance processes or a dinosaur quality management system (QMS) that’s holding the company back. Any of these shortcomings can be problematic for an operation, but the presence of several can be detrimental to the company’s short- and long-term success. It can be a precarious situation to come in as the new ‘gal or guy’ and start changing things right away. In fact, this typically doesn’t bode well for those who take the scorched earth strategy making big changes right out the ‘new hire’ gates, regardless of those changes being warranted. So the question begs to be asked – what do you do if you start a new job and quickly uncover problems within the company’s operations, especially if those problems are compliance related? Taking a measured and strategic approach to your new job and how you will handle the current business practice issues you are experiencing is key to your success. Consider the following process:
Remember that every organization is different, and your approach to addressing poor practices will depend on the specific circumstances. Your ultimate goal should be to contribute positively to the organization's growth and improvement while maintaining your professionalism and integrity. The quickest way to overcome a business challenge is to get help from those who are experienced in besting your beast! The team at Square-1 Engineering is comprised of a variety of technical and project management professionals who are subject matter experts in the areas of NPD, Quality, Compliance and Manufacturing Engineering. Learn more about how we can solve your technical work and project problems today to get you back on track.
0 Comments
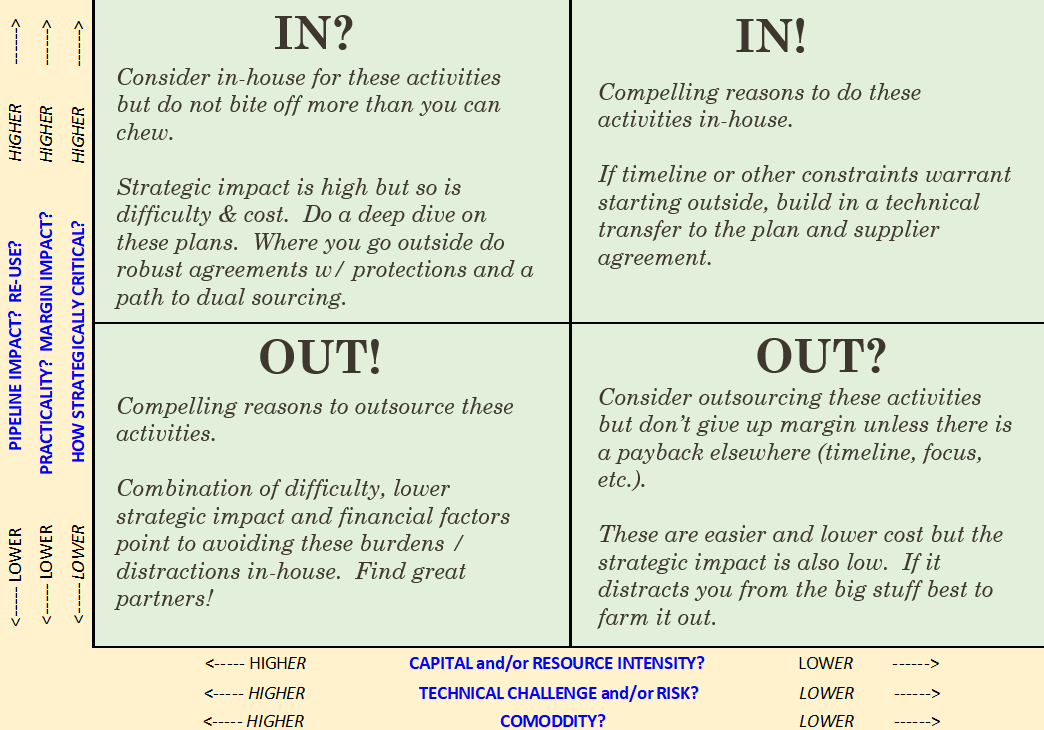
In this Medtech Snapshot episode we cover strategies for supplier management and maximizing operational efficiencies when it comes to getting work done. Listen in as Todd Abraham, medical device chief operating officer, explores creative outsourcing, commodity-based decision making and practical manufacturing operation considerations. Need help making a decision on which tasks to keep internally versus to consider outsourcing? Todd has developed the above decision matrix to help guide the process of identifying when it is appropriate to outsource versus insource a particular task. The more we use tools like this to help in our decision making process the better chance we will have of making the right decision, first time around. #suppliermanagement #operations #medicaldevice #medtech #outsourcing #commodity #manufacturing #operations #snapshot The quickest way to overcome a business challenge is to get help from those who are experienced in besting your beast! The team at Square-1 Engineering is comprised of a variety of technical and project management professionals who are subject matter experts in the areas of NPD, Quality, Compliance and Manufacturing Engineering. Learn more about how we can solve your work and project problems today to get you back on track!
At the forefront of the medical device new product development (NPD) process we typically find a list of considerations we’re trying to address within our product all while coming up with a solution which meets marketplace needs. Talk to anyone in R&D, or downstream marketing for that matter, and you’ll commonly hear the list of ‘needs’ for a new product can seem never-ending. What you may find surprising is how often design for manufacturability (DFM) is not considered in the beginning of the development process. DFM is an important, and often overlooked, part of product development which involves designing a product in a way which makes it easier and more cost-effective to manufacture commercially. While embedding DFM into the process early seems like a logical part of the process, what plays out in reality is an NPD process that is focused on creativity and validating a technology yet can lack appreciation for the future state of the product and it’s foray into the market. So why is it companies may overlook incorporating DFM into the development process? For starters, many of us who design products on the front end, have little perspective, or perhaps no perspective on what it takes to actually produce a scalable product on the back end. Unless you’ve worked on a manufacturing floor or you’ve walked through an entire new product introduction (NPI) cycle, it’s easy to misunderstand all which goes into producing a product to bring to market. The design process also requires creativity and ingenuity in order to find new approaches, break common perceptions of technology capabilities, etc. It’s about ideation! Unfortunately thinking down the road about how it will be built for commercial purposes is “someone else’s problem”. To illustrate this point let’s look towards the automotive industry at a product we all know, which also happens to be a heavily regulated industry, like the medical device industry. If you have ever been to a large-scale automotive show, like the LA Auto Show in Los Angeles, CA, what you’ll see are concept cars on display to wow the eyes and entice the senses. Ironically, if those same concept cars ever got to production, you’ll find their characteristics which made the vehicle look incredible are often diluted down to features and benefits which consumers will accept and are cost efficient to produce. Case in point, the two images below shows a 2010 Chevy Volt electric vehicle. The top image is the concept vehicle – sleek, sophisticated, sharp lines, aggressive character and eye catching. Almost resembles a sports car. The bottom image is the production vehicle – commuter extraordinaire with features which are dull, less angular and more consistent with a car which will be manufactured by the hundreds of thousands. Concept vehicles have their place though – mostly to show off a company’s capabilities and satisfy egos. They just aren’t practical on scale. The truth is if Chevy produced at volume the concept version the average consumer wouldn’t be able to afford it. As a result, they round out the features, slap some plastic on it and Walla you’ve got a commuter vehicle fit for the masses at a price point which coincides with high volume sales. The medical device industry is no different. While industrial design is playing a much larger role today with device design, the fact of the matter is our industry struggles at times with over engineering products just like the auto industry. This can lead to a company losing sight of what the end user needs and wants, as well as if it can actually be manufactured to meet reimbursements. It’s for these reasons it’s important to consider design for manufacturing from the very beginning of the design process. If you do so, you stand to experience the following:
Considering DFM from the beginning of the design process is crucial for optimizing the manufacturing process, reducing costs, improving product quality, and ensuring a smoother path to market. It ultimately results in a more competitive and successful product with potential less risk downstream. The quickest way to overcome a business challenge is to get help from those who are experienced in besting your beast! The team at Square-1 Engineering is comprised of a variety of medical device technical and project management professionals who are subject matter experts in the areas of NPD, Quality, Compliance and Manufacturing Engineering. Learn more about how we can solve your work and project problems today to get you back on track!
Medtech Snapshot is back as we talk with Rohullah Latif who shares his insights on how artificial intelligence (AI) is changing the face of medical device manufacturing. Hear how AI is helping operations become more proactive, while reducing assembly errors. #artificialintelligence #AI #manufacturing #medtech #medicaldevice #snapshot The quickest way to overcome a business challenge is to get help from those who are experienced in besting your beast! The team at Square-1 Engineering is comprised of a variety of technical and project management professionals who are subject matter experts in the areas of NPD, Quality, Compliance and Manufacturing Engineering. Learn more about how we can solve your work and project problems today to get you back on track!
About the AuthorTravis Smith is the founder and managing director of Square-1 Engineering, a medical device consulting firm, providing end to end engineering and compliance services. He successfully served the life sciences marketplace in SoCal for over 15 years and has been recognized as a ‘40 Under 40’ honoree by the Greater Irvine Chamber of Commerce as a top leader in Orange County, CA. Categories
All
Archives
July 2024
|
Visit Square-1's
|
|






 RSS Feed
RSS Feed


