|
We're breaking the mold!
Medtech Snapshot returns with an enticing debate as #RAQA medtech industry experts Stephanie Rallis-Daw, RAC, CQE, CMDA and Robert Lahaderne, MBA spar on the topic of "When starting a new job/ project, what is the most common medtech compliance shortfall you can expect to encounter at your new company?" New to Medtech Snapshot? Check out our archive of past episodes at https://www.sqr1services.com/white-papers/category/snapshot covering topics in R&D, Quality, Clinical and Manufacturing. Honey for your eyes and ears, friends. #medtech #snapshot #podcast #medicaldevice #compliance #quality #regulatory #documentation #training
0 Comments
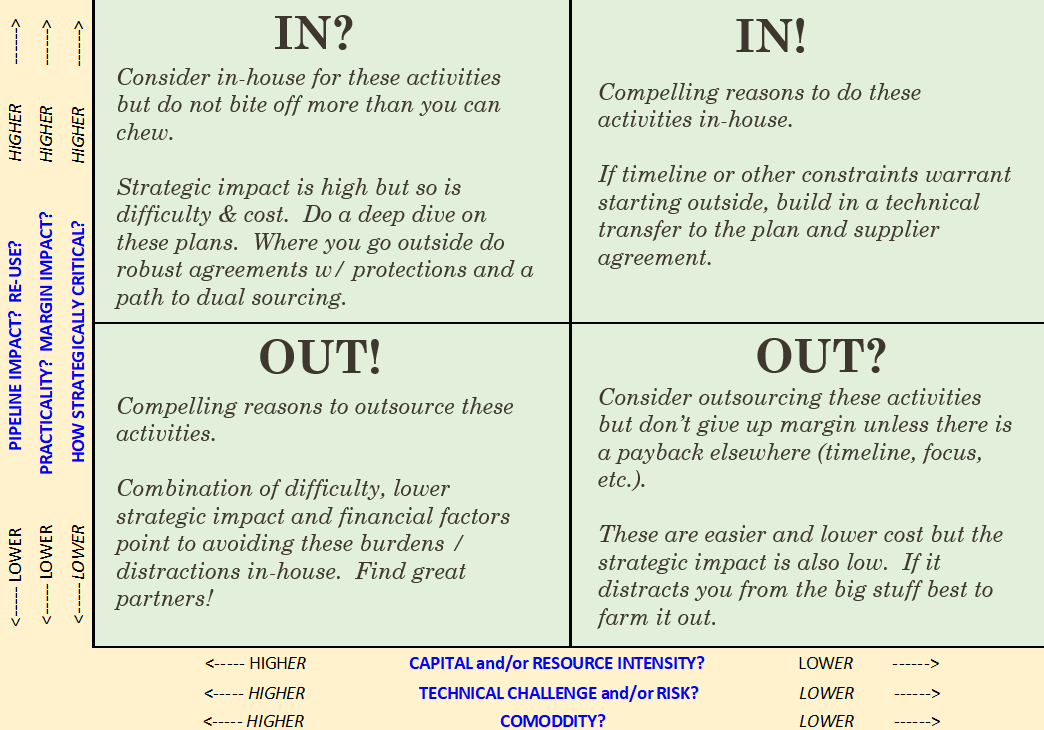
In this Medtech Snapshot episode we cover strategies for supplier management and maximizing operational efficiencies when it comes to getting work done. Listen in as Todd Abraham, medical device chief operating officer, explores creative outsourcing, commodity-based decision making and practical manufacturing operation considerations. Need help making a decision on which tasks to keep internally versus to consider outsourcing? Todd has developed the above decision matrix to help guide the process of identifying when it is appropriate to outsource versus insource a particular task. The more we use tools like this to help in our decision making process the better chance we will have of making the right decision, first time around. #suppliermanagement #operations #medicaldevice #medtech #outsourcing #commodity #manufacturing #operations #snapshot The quickest way to overcome a business challenge is to get help from those who are experienced in besting your beast! The team at Square-1 Engineering is comprised of a variety of technical and project management professionals who are subject matter experts in the areas of NPD, Quality, Compliance and Manufacturing Engineering. Learn more about how we can solve your work and project problems today to get you back on track!
In this week's #medtech #snapshot we're joined by Michelle Caulfield, VP Global Quality, as we talk about medical device data protection and regulatory standards from a cybersecurity perspective. Hear about the considerations for the October 2023 deadline for section 524B, Ensuring Cybersecurity of Devices.
Check out our library of Medtech Snapshot episodes at https://lnkd.in/gFwF9GYN Want to learn more about FD&C Act section 524B, go to: https://lnkd.in/gDeumUcb #cybersecurity #medicaldevice #qualityassurance #qualitycontrol What does it really take?
Today's episode of Medtech Snapshot features our managing director Travis N. Smith as he covers the outcome of an online survey we hosted to learn what people thought 'is the most important characteristic a medical device start-up management team needs in order to successfully take a product to market'. So, the question remains - what does it really take for a management team to bring their product to market? Listen in and find out! If you're enjoying our Medtech Snapshot 3-minute digests be sure to check out our other episodes at https://www.sqr1services.com/white-papers/category/snapshot #medtech #snapshot #medicaldevice #startup #commercialization #product #marketplace #square1engineering #management The world around us is ever-changing and constantly evolving, and for those of us who are leading teams, projects or companies this presents a daily challenge. One area in the business world where we commonly run into change, or scope creep as many of us like to call, is in project work. It doesn’t matter if you are running an internal project, or if you are a consultant operating externally, change in work and projects alike seems constant. If you are new to the term, scope creep is defined as “changes, continuous or uncontrolled growth, in a project’s requirements, at any point after the project begins.” Simply put, it’s a change in plans that was unplanned for after the starting point of a body of work. While complete prevention of scope creep might be unreasonable, employing strategies at the forefront of your project and throughout are vital to ensure you stay on track with as little change along the way. Strategies like the ones listed below also allow the project to ebb and flow so that some change can be accommodated while other changes, which could derail a project, are held off at the proverbial gates. Below you are some strategies to help you prevent scope creep on your next project: 1. Clearly Define the Scope Up Front Have a detailed project scope statement that outlines the project's objectives, deliverables, boundaries, and limitations. Make sure all stakeholders agree on the scope before starting the project. Special note – want to get approval quickly for your project? Make sure the project and intended outcomes align with the company’s mission and primary objectives while clearly showing a good return on investment (ROI). 2. Engage Stakeholders From the Beginning Involve key stakeholders in the scope definition and planning processes. Their input and feedback can help identify potential scope creep early on and ensure that their expectations align with the project's scope. 3. Set Realistic Goals Establish achievable project goals and objectives. Unrealistic expectations can lead to scope creep as stakeholders try to add additional features or requirements. If you can’t get agreement on the goals of the project this should cause you to pause before moving forward. Remember, each person involved may have a different perspective of what is needed based on their own biases. 4. Create a Change Control Process Develop a formalized process for requesting and approving changes to the project scope. I like to use the engineering change order (ECO) process on our projects as it formalizes changes and requires approval from stakeholders before the changes are enacted. All changes should go through this process, involving the evaluation of their impact on time, cost, and resources before being accepted. Speaking of scope creep, in this episode of Medtech Snapshot Terry Vick, Sr. Director Program Management at Shockwave Medical out of Santa Clara, CA talks about some of the strategies in this list which we need to be aware of and consider upfront in the project planning phase into order to limit scope creep on the back end. Plus, the importance of having an internal project sponsor. Back to our list of strategies to help you prevent scope creep… 5. Prioritize Requirements Use techniques like MoSCoW (Must have, Should have, Could have, Won't have), Five Whys or the Analytic Hierarchy Process (AHP) to prioritize project requirements. This helps in focusing on what's essential and avoids unnecessary additions. 6. Track Progress Against Scope Regularly track project progress against the defined scope. Tools like Microsoft Project which build out real-time Gannt charts are incredibly helpful to visualize tasks and progress. This also helps identify any deviations early on and allows for timely corrective actions. 7. Communicate to Your Team Consistently & Clearly Ensuring all team members and stakeholders understand the project's scope and the implications of scope changes is paramount to the success of the project. Effective communication can help prevent misunderstandings that might lead to scope creep. 8. Document Changes to the Project Keep a detailed record of all changes to the project scope, including their rationale and impact. This documentation helps maintain transparency and accountability. 9. Manage Expectations by Providing Project Updates to Stakeholders Continuously manage stakeholder expectations by providing regular updates on project status and any changes to the scope. This can help prevent unrealistic demands and last-minute additions. 10. Institute a Review and Approval Process for Scope Change Institute a formal review and approval process for scope changes. All changes should be evaluated by relevant stakeholders and approved based on their impact. 11. Stay Firm but Flexible While it's important to resist unnecessary scope changes, be open to valid suggestions or changes that genuinely enhance the project's value. Just ensure that these changes go through the proper evaluation and approval processes. If you can’t readily identify the ROI of the change it probably isn’t a good change to consider. 12. Conduct Regular Project Reviews Conduct regular reviews with stakeholders to ensure that the project is meeting their expectations and to address any concerns before they escalate. When facilitating meetings, try to employ Jeff Bezos’ ‘Two Pizza’ rule when it comes to meeting attendees. 13. Manage Dependencies to Avoid Unintended Cross Functional Impact Understand and manage dependencies between project tasks and deliverables. Changes in one area can often impact other parts of the project. Remember that while complete prevention of scope creep might be challenging, these strategies can significantly help to reduce its occurrence and impact on your project. Regular vigilance and proactive management are key to avoiding scope creep from taking over your project. The quickest way to overcome a business challenge is to get help from those who are experienced in besting your beast! The team at Square-1 Engineering is comprised of a variety of technical and project management professionals who are subject matter experts in the areas of NPD, Quality, Compliance and Manufacturing Engineering. Learn more about how we can solve your work and project problems today to get you back on track! In this episode of Medtech Snapshot we hear from Karen Polkinghorne, President of Network Partners Group, on her three strategic considerations for improving your chances of gaining approval with management on the new project, program or initiative you're trying to get under way. Learn how the companies vision and mission, as well as the return on investment (ROI) for your project, plays an important role in the approval process. #medicaldevice #medtech #snapshot #approval #project #program #management #returnoninvestment #vision #mission Get Medical Device Project Support TodayThe quickest way to overcome a business challenge is to get help from those who are experienced in besting your beast! The team at Square-1 Engineering is comprised of a variety of technical and project management professionals who are subject matter experts in the areas of NPD, Quality, Compliance and Manufacturing Engineering. Learn more about how we can solve your work and project problems today to get you back on track!
What we know to be true is root cause analysis (RCA) plays a vital role in the development, manufacturing, and use of medical devices. When we are able to successfully identify and address the underlying causes of failures or errors, using RCA as a tool in your operation helps to improve the safety, reliability, and effectiveness of medical devices. When done correctly, RCA has a significant impact on medical devices by enhancing patient safety, improving design and manufacturing processes, ensuring regulatory compliance, driving product improvement and iteration, mitigating risks, and supporting post-market surveillance efforts. While all of this may sound common sense, what's most important is when RCA is used and done correctly. In this episode of Medtech Snapshot we're joined by Quality Executive Robert Lahaderne to talk Root Cause Analysis (RCA)! Hear how Robert shares his perspective on the impact Root Cause makes to a medical device organization when it is done correct versus when it is done incorrectly and how rushing through FMEAs and hazard analysis creating unnecessary challenges down the road. #rootcauseanalysis #rootcause #medicaldevice #medtech #fmea #hazardanalysis #patientsafety #riskmanagement Get Medical Device Project Support TodayThe quickest way to overcome a business challenge is to get help from those who are experienced in besting your beast! The team at Square-1 Engineering is comprised of a variety of technical and project management professionals who are subject matter experts in the areas of NPD, Quality, Compliance and Manufacturing Engineering. Learn more about how we can solve your work and project problems today to get you back on track! When you’re early in the development process it can seem like there’s a never ending list of activities and projects your R&D team needs to take on. When you’re in this mode its vital R&D leaders assess time, resources and associated risks with the product they’re developing. Medical device product development executive Arif Iftekhar walks us through how to focus your team and address the most important product risks head on at an early stage. In this episode of Medtech Snapshot we discuss strategic supply chain strategies with medical device supply chain and manufacturing executive Jeff Brown. Key points in this discussion include addressing situations similar to China and Taiwan, solutions to hedge yourself against such global challenges, including but not limited to supplier risk, back-up capacity and vertical integration. Need help sorting through your supply chain and or contract manufacturing challenges? Learn more about Square-1 Engineering's capabilities as it relates to all things manufacturing engineering: NPI, tech transfer, pilot manufacturing, process development and improvement, supply chain management, etc. Click HERE to learn more.
This video covers our managing director Travis Smith and Arif Iftekhar, VP R&D, discussing the outcome of Square-1 Engineering's recent online poll covering a crucial product development question: What is the best way to predict medical device product failures on the bench before entering clinical trials? Other notable comments from this online poll included: Glen Rabito - Director Engineering: "get real about your pre-DV testing, it's not a check the box exercise. Perform as much pilot clinical work as time and money allows prior to starting your trial." Jason Safabash - VP R&D: "Ensure inputs accurately reflect device safety and efficacy requirements and test early/ fail fast and feedback into development to reduce the risk of failures during verification or validation testing." Jahnavi Lokre - COO: "definition and understanding of clinical user needs is key to the success of a clinical trial. Couple with a robust risk management process and pre clinical testing will provide confidence on the safety and efficacy of your medical device." For more poll insights go to: https://www.linkedin.com/feed/update/urn:li:activity:7036389649199636480 About the AuthorTravis Smith is the founder and managing director of Square-1 Engineering, a medical device consulting firm, providing end to end engineering and compliance services. He successfully served the life sciences marketplace in SoCal for over 15 years and has been recognized as a ‘40 Under 40’ honoree by the Greater Irvine Chamber of Commerce as a top leader in Orange County, CA. Categories
All
Archives
April 2024
|
Visit Square-1's
|
|





 RSS Feed
RSS Feed


