|
In this 2-part series of Medtech Snapshot we're joined by Square-1 Engineering Director of Delivery & Operations Trisha Aure as she walks us through the highlights of our article 'FDA Announcement: 21 CFR 820 and ISO 13485 Guidance'
Hear the areas where 21 CFR 820 differs most from ISO 13485 and what this means for medical device OEMs. Part-2 of this series will cover the process to transition and key considerations when doing so. #iso13485 #regulation #compliance #fda #21CFR #riskmanagement #medicaldevice #medtech #news #podcast #snapshot #regulatoryaffairs
0 Comments
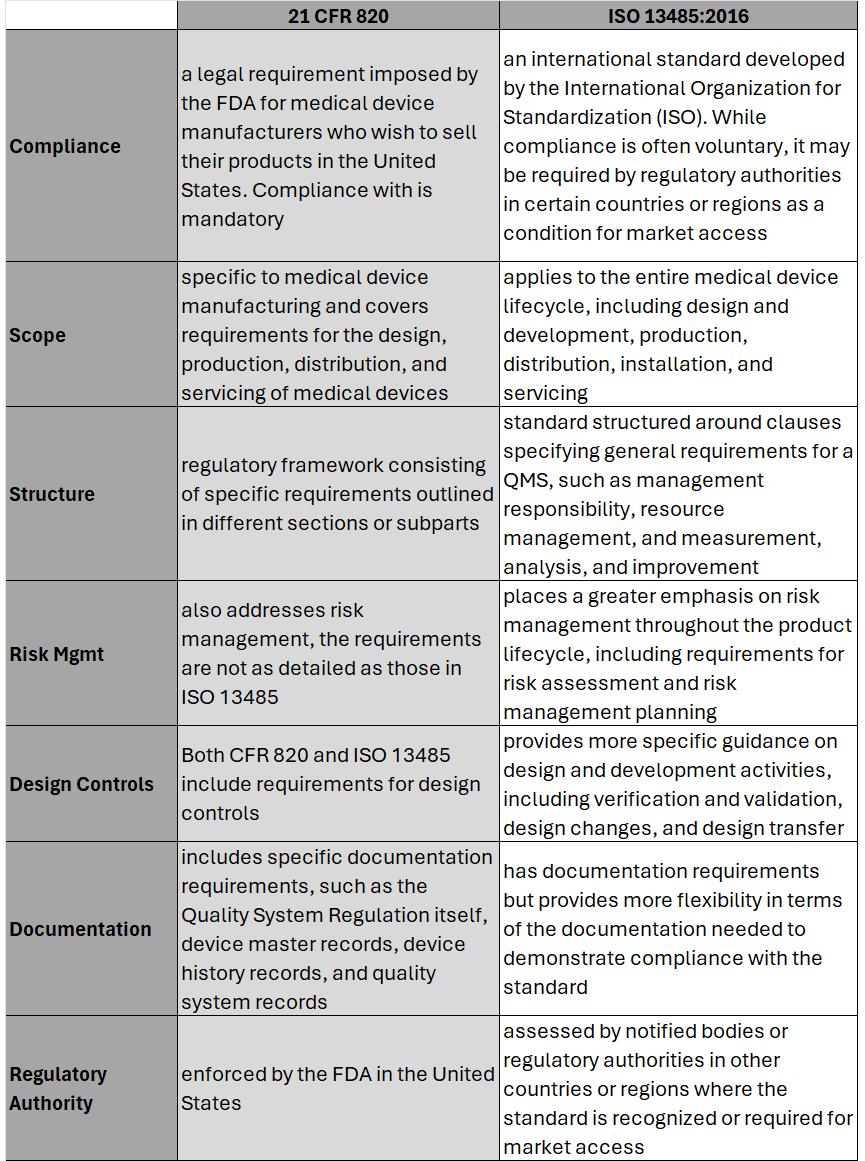
On February 2nd, 2024 the U.S. Food and Drug Administration (FDA) made its formal announcement and ruling providing guidance on 21 CFR Part 820 which up to that point was the standard overseeing medical device quality system regulation and current good manufacturing practices (cGMP) in the United States of America. The big news – 21 CFR 820 will be heavily amended to incorporate ISO 13485 as the leading guidance for Quality Management System Regulation (QMSR) and cGMP. While the news wasn’t a surprise, it does put a final note on the direction the agency intends to take for medical device practices moving forward, especially as it relates to risk management. While many device OEMs already utilize ISO as their leading regulation standard, those who don’t will have two years to adjust to these changes to be in compliance effective February 2nd, 2026. Here’s what you need to know as it relates to the differences between 21 CFR 820 and ISO 13485, as well as considerations OEMs should take into account in order to meet the 2026 deadline. Big Picture:
Key Differences: Transitioning to ISO 13485 Transitioning from CFR 820 to ISO 13485 involves several steps to ensure compliance with the ISO standard. A general outline of the steps an OEM should take to transition may include: 1. Understand the Requirements of ISO 13485: Familiarize yourself with the requirements of ISO 13485. This includes understanding the structure of the standard, its key clauses, and any specific requirements which may differ from CFR 820. (see above table for highlights) 2. Gap Analysis: Conduct a thorough gap analysis to identify the differences between your current quality management system under CFR 820 and the requirements of ISO 13485. This will help you determine what changes, if any, need to be made to your existing processes, procedures, and documentation. This is also a great time to do a review of your QMS tool to determine if it is an appropriate tool for future use. 3. Document Review and Update: Review your existing documentation, including quality manuals, procedures, work instructions, and forms, to ensure they align with the requirements of ISO 13485. Update or create new documents as necessary to meet the standard's requirements. 4. Training and Awareness: Provide training to relevant personnel to ensure they understand the requirements of ISO 13485 and their roles in implementing and maintaining the QMS. This may include training on new procedures, processes, and documentation. 5. Implementation of New Processes: Implement any new processes or procedures required by ISO 13485. This may include processes related to risk management, design and development, purchasing, production, and service provisions. 6. Internal Audits: Conduct internal audits of your QMS to verify compliance with ISO 13485 requirements. Identify any non-conformities and take corrective actions to address them. 7. Management Review: Hold management reviews to evaluate the effectiveness of the QMS and identify opportunities for improvement. Ensure top management is actively involved in the transition process and committed to maintaining the QMS. 8. Certification Audit: BEFORE you consider this step be sure to speak with a regulatory affairs subject matter expert to ensure it is necessary. Once you believe your QMS is fully compliant with ISO 13485, engage a certification body to conduct a certification audit. The audit will assess your organization's compliance with the standard and determine if you are eligible for certification. 9. Address Non-conformities: If any non-conformities are identified during the certification audit, take corrective actions to address them. The certification body will typically require verification that corrective actions have been implemented before issuing the ISO 13485 certificate. 10. Continual Improvement: Continuously monitor and improve your QMS to ensure ongoing compliance with ISO 13485 and to enhance the efficiency and effectiveness of your processes. Although the 21 CFR 820 and ISO 13485 vary in their structure, and at times use different terminology to describe similar concepts, 21 CFR 820 and ISO 13485 are substantially similar in that both prioritize principles such as risk management, design controls, and continual process improvement. It’s possible as organizations begin to look at their current standards and systems, they will find the transition process is not as cumbersome as initially thought. While this is an obvious assumption, it’s important to note regulatory affairs professionals should be counseled throughout this entire process to ensure appropriateness of adoption and change management. Medtech Snapshot: Forward Progress in Product Development with Glen Rabito, COO of Nidus Biomedical1/10/2024 Medtech Snapshot episode #21 features Nidus Biomedical COO Glen Rabito talking through the five considerations to keep product development moving forward, especially in the early phases of development.
#medtech #snapshot #medicaldevice #productdevelopment #NPD #riskmanagement #userneeds #patientsafety Medical device companies play a critical role in advancing healthcare as their ability to diagnose, monitor, and treat medical conditions allow patients like you and I the opportunity to recover and live longer. Device companies carry a heavy burden on our behalf and that burden starts with product risk. One of the biggest challenges an OEM medical device organization will be faced with is managing risk, especially during the early stages of product development. The integration of risk management into design control (ISO 14971) is essential for identifying, assessing, and mitigating potential risks associated with the design and development of a medical device. Given risk management is a part of nearly every development process, and is a primary focus of all regulatory agencies like the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA), why is it then so many medical device companies struggle with sound risk management strategies? The failure to address risks adequately can lead to a whole host of problems ranging from regulatory non-compliance, compromised patient safety, financial setbacks, and in severe situations criminal prosecution of executives. Needless to say, understanding why medical device companies come up short with their risk management strategy and how you can avoid that for yourself is key to your future success. In this article, we will explore some of the key reasons behind risk management failure. Most Common Risk Failure Factor - Inadequate Understanding of Regulatory Requirements: One of the primary reasons for failure in risk management is an insufficient understanding of the complex and evolving regulatory landscape. Medical device companies must navigate a web of regulations, standards, and guidelines to ensure compliance. Failing to keep abreast of these requirements can result in flawed risk assessments, inadequate risk mitigation measures, and ultimately, regulatory sanctions. Poor Integration of Risk Management into Product Development: Successful risk management should be an integral part of the product development lifecycle. However, some companies make the mistake of treating it as a standalone process rather than integrating it seamlessly into every stage of development. When risk management is an afterthought, essential risks may be overlooked, leading to suboptimal product designs, increased failure rates, and compromised patient outcomes. Lack of Cross-Functional Collaboration: Effective risk management requires collaboration across various departments, including research and development, regulatory affairs, quality assurance, and manufacturing. Failure to establish clear communication channels and encourage cross-functional collaboration can result in siloed decision-making. This lack of coordination can lead to critical risks being underestimated or missed entirely. Insufficient Resources and Expertise: Some medical device companies fail in risk management due to resource constraints and a shortage of expertise. This can manifest in inadequate training for personnel responsible for risk management, insufficient allocation of time and budget, and a lack of access to external expertise. Without the necessary resources, companies may struggle to conduct comprehensive risk assessments and implement effective risk mitigation strategies. Overemphasis on Short-Term Goals: Pressure to meet short-term financial goals can sometimes lead companies to prioritize speed to market over thorough risk analysis. This can result in hasty decision-making and inadequate risk identification and mitigation. Companies need to strike a balance between meeting market demands and ensuring the safety and efficacy of their medical devices in the long run. Failure to Learn from Industry Incidents: The medical device industry has witnessed several high-profile incidents related to product failures and patient harm. Failure to learn from these incidents and implement lessons learned into future risk management strategies can perpetuate the same mistakes. Companies must actively analyze industry incidents, update risk management processes accordingly, and continuously improve their practices. Ineffective Communication with Stakeholders: Communication is crucial in risk management, both internally and externally. Companies that fail to communicate effectively with their stakeholders, including regulatory bodies, healthcare professionals, and patients, may face increased scrutiny and regulatory challenges. Transparency and open communication are essential for building trust and demonstrating commitment to patient safety. In the highly regulated and dynamic field of medical devices, effective risk management is not just a regulatory requirement - it is a fundamental aspect of ensuring patient safety and the success of a company. Understanding the pitfalls that lead to failures in risk management can help medical device companies proactively address these challenges. By prioritizing compliance, integrating risk management into every stage of product development, fostering cross-functional collaboration, and learning from industry incidents, companies can enhance their risk management strategies and contribute to the advancement of healthcare with safe and reliable medical devices. The quickest way to overcome a business challenge is to get help from those who are experienced in besting your beast! The team at Square-1 Engineering is comprised of a variety of technical and project management professionals who are subject matter experts in the areas of NPD, Quality, Compliance and Manufacturing Engineering. Learn more about how we can solve your work and project problems today to get you back on track!
What we know to be true is root cause analysis (RCA) plays a vital role in the development, manufacturing, and use of medical devices. When we are able to successfully identify and address the underlying causes of failures or errors, using RCA as a tool in your operation helps to improve the safety, reliability, and effectiveness of medical devices. When done correctly, RCA has a significant impact on medical devices by enhancing patient safety, improving design and manufacturing processes, ensuring regulatory compliance, driving product improvement and iteration, mitigating risks, and supporting post-market surveillance efforts. While all of this may sound common sense, what's most important is when RCA is used and done correctly. In this episode of Medtech Snapshot we're joined by Quality Executive Robert Lahaderne to talk Root Cause Analysis (RCA)! Hear how Robert shares his perspective on the impact Root Cause makes to a medical device organization when it is done correct versus when it is done incorrectly and how rushing through FMEAs and hazard analysis creating unnecessary challenges down the road. #rootcauseanalysis #rootcause #medicaldevice #medtech #fmea #hazardanalysis #patientsafety #riskmanagement Get Medical Device Project Support TodayThe quickest way to overcome a business challenge is to get help from those who are experienced in besting your beast! The team at Square-1 Engineering is comprised of a variety of technical and project management professionals who are subject matter experts in the areas of NPD, Quality, Compliance and Manufacturing Engineering. Learn more about how we can solve your work and project problems today to get you back on track! Recently our company published a poll online offering up the following question for the medical device community: “What is the most important factor to consider when developing a medical device product?” At the close of the poll dozens of people had cast their votes for what they believed to be the factors affecting product development the most. The poll options included:
If you have been in industry for any length of time you know there are dozens of factors which can and often have a direct impact on the product development process. While there are dozens to consider, such as PRS (Product Requirement Specification), planning, user experience, DFM, etc. what we know to be true is each of these factors carry varying weights of impact. They are not all equal in measure or influence. As our poll launched and picked up steam one of the four factors listed as an option began to take a commanding lead. The respondents, who are largely made up of medical device professionals and executives, had identified a common factor which stood above the rest in its ability to impact positively or negatively the product development process. What was this most important factor? Would you have guessed ‘Having the Right Team in Place’ is the number one factor which determines success when developing a medical device product? ‘Having the Right Team in Place’ was identified by 51% of the respondents as being the most important factor which directly contributes to the success of medical device product development. The other options broke down as follows: Simply put – having the right team in place covers all of the other areas that potentially could produce challenges during the product development cycle. Whereas the inverse is certainly all too true. When we have the wrong team in place, or teammates lacking the capabilities to facilitate their job as needed by the company, inevitably problems go arise which hold back otherwise good opportunities and technology offerings. Jim Collins, celebrated author (books like ‘Good to Great’ & ‘Built to Last’) and business management guru, is quoted as saying “Leaders of great companies start by getting the right people on the bus, the wrong people off the bus, and the right people in the right seats.” What this means is it’s more about the people than it is the technology or problem you’re solving. This is an important lesson, especially for first time entrepreneurs and startup executives. You can have the best product idea in the world, one that is in high demand, but if you don’t have the right team in place you’ll most likely spin your wheels while blowing out copious amounts of money in the process. We’ve also seen this reality in person dozens of times. As a medical device consulting firm we work with a lot of companies, both start up and conglomerate alike. One of the consistent characteristics we see within the companies which are able to drive success, often times repeated success, is their management team is comprised of experts in their particular field who know how to both lead and operate in the weeds. They both strategic and tactical, able to plan for the long term while addressing todays shorter term needs. As a result, they know how the job is done and therefore can either lead or delegate those tasks helping to guide their department or team to successful completion. When you have the right people on the team (your bus) you will then find opportunities (the medical problem you’ll solve) to move forward with. Following this process you’ll also have a far better chance of facilitating that opportunity through the development process and into commercialization, or acquisition. About the AuthorTravis Smith is the founder and managing director of Square-1 Engineering, a medical device consulting firm, providing end to end engineering and compliance services. He successfully served the life sciences marketplace in SoCal for over 15 years and has been recognized as a ‘40 Under 40’ honoree by the Greater Irvine Chamber of Commerce as a top leader in Orange County, CA. Categories
All
Archives
July 2024
|
Visit Square-1's
|
|







 RSS Feed
RSS Feed


